THE RUSSIAN FEDERATION MINISTRY OF PUBLIC HEALTH MOSCOW FIRST STATE MEDICAL UNIVERSITY
named after I. SECHENOV
Not for publication
NATALIA P. MIKHAYLOVA
Clinical and experimental study of the influence of intradermal injection of modified hyaluronic acid on the morfofunctional condition of skin with involutional changes
14.01.10 - Skin and Venereal Diseases Ph.D. Thesis in Medicine
Research Supervisors: N.N. Potekayev, Professor, M.D.;
A.B. Shekhter, Professor, M.D.
Moscow, 2014
Table of Contents
ABBREVIATIONS
Introduction
CHAPTER 1. Literature Review
1.1. Aging as a biological and social problem
1.2. Aging theories description
1.3. Main principles of geropreventive therapy
1.4. Contemporary views of skin aging
1.5. Injection methods of treating aging skin change in aesthetic medicine
1.6. Hyaluronic acid in anti-aging skin therapy
CHAPTER 2. Materials and methods
2.1. General characteristics of the experimental studies
2.2. General characteristics of the examined patients
2.3. Non-invasive methods for assessing morphological and functional parameters of patients' skin
2.4. Methods for injecting modified hyaluronic acid
2.5. Methods of statistical processing of results
CHAPTER 3. RESULTS AND DISCUSSION
3.1. RESULTS OF A MORPHOLOGICAL STUDY OF SUBCUTANEOUS INTRODUCTION OF HYALURONIC ACID GELS UNMODIFIED AND MODIFIED WITH VITAMINS, AMINO ACIDS AND OLIGOPEPTIDES
3.1.1 Results of macroscopic examination of subcutaneous gel injection sites
3.1.2. Histological and histochemical study of tissue reaction and resorption of gel implants
3.2. RESULTS OF A HISTOLOGICAL STUDY OF INTRACUTANEOUS INJECTION OF UNMODIFIED AND MODIFIED HYALURONIC ACID GELS
3.2.1. Results of histological and histochemical study
3.2.2. Results of semi-thin sections study
3.2.3. Transmission electron microscopy
3.3. STUDY OF CLINICAL AND MORPHO-FUNCTIONAL SKIN PARAMETERS IN PATIENTS OF DIFFERENT AGES BEFORE AND AFTER INJECTIONS OF MODIFIED HYALURONIC ACID
3.3.2. Influence of modified hyaluronic acid injections on skin elasticity
3.3.3. Changes in the skin’s functional characteristics – moisture, pH, and greasiness
3.3.4. Evaluation of change in skin microtopography by ultrasound scanning
3.3.5. Evaluation of skin microrelief changes
3.3.6. Evaluation of skin microtopography by confocal laser scanning microscopy
Conclusion
Findings
Practical recommendations
AOD: antioxidant defense
BAS: bioactive substances
DNA:
desoxyribonucleic acid
DE: diglycidyl ethers
GAG:
glycosaminoglycans
HA: hyaluronic acid
IL: interleukins
BMI: body mass index
CLSM: confocal laser scanning
misroscopy
H&E: hematoxylin and eosin staining
LP: lipid
peroxidation
PG: prostaglandines
RNA: ribonucleic acid
FRO: free-radical oxidation
UVR: ultraviolet radiation
MMP: matrix metalloproteinases
Rationale
It can be said with confidence today that among the numerous methods used for skin rejuvenation in aesthetic medicine and cosmetology, special attention is being paid to injection techniques for correction of involutory skin changes, by both doctors and patients. Also, there is a recent trend for constant growth of injection therapy methods to correct involutory skin changes. According to a 2009 report of the American Society for Aesthetic and Plastic Surgery (ASAPS), hyaluronic acid (HA) injections take second place among the other non-invasive methods of skin rejuvenation (1,313,038 HA injections in the USA, 2009), ranking next to botulotoxinum A injections only (2,557,068 injections in the USA, 2009) (125).
Skin aging, as a subcase of the entire body's aging, can be considered with regard to changes in the stationary state where complex compounds like collagen, elastin, acid glycosaminoglycans (GAG), particularly hyaluronic acid (HA), are being continuously synthesized in one kind of processes and decomposed in others. The processes of synthesis and decomposition should be maintained coordinated in order for the skin to be healthy (145). Thus, for instance, there is a strict correlation between the intensivity the exchange of GAGs, their protein complexes (proteoglycans) and collagen proteins of the dermis. It is the correlation between these processes that the intensivity of skin aging depends on. With age, or because of environmental damage, this correlation changes as biomolecules of this type start forming slower and decomposing faster (22). With this regard, the strategy to be implemented in injection cosmetology should be intended to create a physiologically beneficial environment to increase skin cells’ metabolic activity, thereby boosting the synthesis of the main components of the dermis’ intercellular matrix (71). Dermatological practice includes an experience of introducing monopreparations based on HA, both native and partly stabilized (82, 109). Initially, hyaluronic acid in injection dermatology served as a filler for intercellular space, but the development of experimental and theoretical biophysical methods of polymer studies boosted further exploration of HA as a natural regulator of certain biological processes (fibroblast migration and epithelical cells proliferation, stimulation of angiogenesis, activation of homeostasis components, etc.). HA has also been recognized as one of adaptogenes-syntoxins and an efficient biologically active substance (72). Also, HA has been recognized as transport for delivery and controlled release of medicines (74). Thanks to the development of new technologies, there is now an opportunity to create preparations of HA modified with vitamins, microelements, amino acids and oligopeptides with geroprotective, particularly antioxidant, action. It resulted in the creation of products to treat involutory skin changes with HA and vitamin C, glycine, proline, lysine, valine, cysteine, methionine, glutatione, etc. (72). It is known from literature that vitamin C can influence the formation of GAGs, particularly HA and chondroitin sulfate, and stimulate the proliferation of fibroblasts (141). The physiological effect of vitamin C is attributed not only to the stimulation of collagen production but also with decreased production of metalloproteinases, the enzymes that destroy dermal collagen. Many works confirm that ascorbic acid (AA) is able to improve skin condition, keep it healthy, particularly by counteracting initial signs of aging (54). The aminoacids glycine, proline, lysine, valine form part of the basic proteins of the dermis’ intercellular matrix. They should be present in injection products together with other low-molecular bioregulators and microelements in order for the body to launch its own collagen and elastin production, which is of utmost importance for a stable and long-term effect. The sulfur-containing aminoacids cysteine, methionine and the tripeptide glutation are very powerful antioxidants that act at different stages of the free-radical chain process of biomolecules oxidation (41). In light of the above, the scope and objective of the present study are as follows.
Scope of study
Study the influence of intradermal injections of BAS-modified HA on the morphofunctional condition of skin.
Study objectives:
1. Study the influence of intradermal and subcutaneous injections of modified HA on the morphological structure and ultrastructure of skin in an animal experience (rats and minipigs)
2. Perform clinical evaluation of the efficiency and tolerability of modified HA in persons with involutory skin changes.
3. Study the functional parameters of skin with involutory changes in the proces of intradermal injection of modified HA.
4. Assess the effect of modified HA over the structural components of skin with involutory changes.
Rationale
This is the first time when the influence of HA modified with biologically active substances (BAS) on the morphological structure and ultrastructure of skin is studied on an animal model with rats and minipigs, in real time mode for subcutaneous and intradermal injection.
It has been shown for the first time that BAS-modified HA is a biocompatible and biologically inert material with a higher clinical efficiency than that of native (non-modified) HA.
It has also been shown for the first time that the injection of HA gels modified with vitamin C (per se and with amino acids), oligopeptides and folic acid activates the proliferation of fibroblasts and neoangiogenesis, boosts the synthesis and fibrillogenesis of collagen and elastin, however without creating a connective tissue capsule around the implant.
For the first time, we assessed the effect of modified HA over the structural components of skin with involutory changes.
We prove for the first time that intradermal injections of Ha-based products modified with such biologically active substances as vitamins, amino acids, and oligopeptides, creates a physiologically favorable environment for boosting skin cells’ metabolic activity, resulting in the activation of the synthesis of basic dermal intercellular matrix components, promoting skin rejuvenation, reducing its involutory changes and improving its functional parameters.
A clinical assessment has been performed with regard to the efficiency and tolerability of modified HA in persons with involutory skin changes. This makes it possible to use such products for combination therapy of involutory skin changes.
Practical value
This is the first time when intradermal injection of modified HA covalently bonded to biologically active substances is used to correct involutory skin changes in persons of various age groups. The method’s outstanding efficiency and tolerability have also been shown. The results obtained can be used to develop pathogenetic plans of treating external aging signs in elderly and senile patients, thereby dramatically improving their life quality.
Theses submitted for discussion
– Preparations of HA modified with vitamins, amino acids and oligopeptides have outstanding biocompatibility and tolerability, are gradually resorbed and do not result in the formation of connective tissue capsules at the point of injection and also cause no adverse skin reactions.
– Intradermal injection of modified hyaluronic acid results in dramatic improvement of the skin’s regeneration potential and reduces the outer manifestations of aging involution.
– Injection of products from BAS-modified HA is a new, promising and highly efficient method of treating involutory skin changes.
Approval of the study
The materials of this thesis were presented and discussed at the 1st Moscow Forum “Dermatolvenereology and Cosmetology: Science Meeting Practice” (Moscow, October 2010), 11th All-Russia Congress of Dermatovenereologists (Yekaterinburg, November 2010), 3rd International Medicine and Beauty Forum (Moscow, December 2010), the 10th International Symposium for Aesthetic Medicine (Moscow, January 2011), Practical Forum “Educational Days for Aesthetic Medicine and Cosmetology Professionals” (Alma-Ata, April 2011), the International Conference of the Ukraine Society for Aesthetic Medicine
“Aesthetic Medicine: Challenges and Solutions. Standardization of Approaches to Aging Changes Treatment” (Odesa, June 2011), 11th International Aesthetic Medicine Symposium (Moscow, January 2012), the 2nd Congress of the Euro-Asian Dermatovenereologists Association, the 5th International Medicine and Beauty Forum (Moscow, March 2012), Estet Beauty Expo-2012 Beauty Industry Congress (Kyiv, april 2012), the Beauty Medicine Forum (Yekaterinburg, June 2012), the 15th International Congress on Neuron Capture Therapy (10-14 September 2012, Tsukuba, Japan), 2nd Moscow Forum “Dermatovenereology and Cosmetology: Science Meeting Practice” (Mosow, October 2012).
The thesis was approved at the Joint Science and Practice Conference of the Skin Reparative Processes Laboratory of the R&D Center and the Department for Skin and Venereal Diseases of Moscow First Medical University named after I.M. Sechenov.
Practical implementation
The practical results have been implemented in the work of the Skin Reparative Processes Laboratory of the R&D Center and the Department for Skin and Venereal Diseases of Moscow First Medical University and are also used in the therapeutic practice of the Reforma Aesthetic Medicine Clinic.
Publications
There are 25 published works on the subject of this thesis, 5 of them in peer-reviewed journals.
Structure of thesis
The thesis consists of 134 typewritten pages, including an introduction, literature review, description of study materials and methods, results of discussion, conclusion, findings, and list of references. The bibliographic index includes 173 sources, of them 83 Russian and 90 from other countries. The thesis is illustrated with 57 figures and 14 tables.
1.1. Aging as a biological and social problem
Aging is an important problem of medical and socioeconomical nature. In view of the growing average life expectancy, the global demographic structure is undergoing dramatic changes. According to ONU forecasts, the share of persons aged 60 and above will have doubled by 2050 in the general population and grown by 4 times in growing economies. This means that society is expected to create an environment for elderly people to participate efficiently in all life spheres (1, 48). The advances in molecular biology and genetics have expanded our understanding of the mechanics of aging and allowed us to successfully fight the main diseases known to reduce life expectancy. As part of the healthy aging concept, measures intended to improve active life quality turn out to be very relevant as well. First and foremost, it is about maintaining and preserving young-looking and attractive appearance, which will definitely benefit the contribution of elderly people to the development of society.
1.2. Aging theories description
Notwithstanding the existing advances in herontology, there is currently no generally accepted concept of aging. However, there are over 100 hypothesis regarding the causes behind aging. These can be divided into stochastic (random-related) theories and programmed aging hypothesis. Theories are also classified in terms of live matter organization level and other parameters. It should be noted that this division is to a great extent conventional, since all these mechanisms are equally important and interrelated. To our belief, the most adequate classification divides theories into three groups: genetic, damage accumulation theories and synthetic theories.
The first group sees aging as a genetically programmed process (1, 11, 43). This point of view is based on the discovered regularities of the time when various aging changes in cell structure show up, such as translational activity decrease, increased activity of hystone acetylation and methylation, appearing of new ribonucleic acids (RNA) fractions and new antigen proteins, activation of mechanisms resulting in cell apoptosis (programmed death). This group can include A. Olovnikov’s telomeric aging hypothesis (44), the theory of cellular (replicative) aging (124), apoptosis theory (21, 75), and also theories of cellular death genes existence, including gene p53, gene bcl-2, genes of apopyloprotein-E (ApoE), and the angiotensine converting enzyme (ACE) (84). Some modern aging theories see DNA changes as the main cause of aging cell changes, like the somatic mutation theories and the reparation systems decreased efficiency theories (1). It has been proven that average life expectancy is no more than 25% due to genetic factors (70), unlike the maximum life expectancy. Still, it is also defined by genetic dispositions for just 30-40%, while uninherited factors determine 60-70% of it (103). Therefore, it is just as important to understand which specific cell damage tends to accummulate over age.
The second group includes aging theories that consider an organism’s death as a result of being “worn out” by autotoxication or environmental damage throughout the life of this organism. The free radical theory, the best-known of them, suggested by D. Harman, supposes that the causes of aging changes consist in the accummulation of damage in all structural components of cells through free radicals (7, 9, 14, 29, 47, 122). The glycation theory has also been gaining popularity lately. According to it, glycation reactions increase the body concentrations of toxic substances that correlates with biological age (155). The main principle of immunological theories is the decreased ability of the immunocompetent cells to recognize extraneous antigens and increased capability of reacting with the body’s own antigens, which results in chronic autoimmune conflict (49). The hypothesis that gut flora could be involved in involutory changes (the autointoxication theory) was initially presented by I. Mechnikov. In the last years, gut flora endotoxin was proven to participate in the pathogenesis of certain diseases in elderly people (2, 31).
The third group of theories comprises the so-called synthetic theories that represent aging as a multi-faceted process of interaction between genetic factors and environment (34, 38, 50, 58). The best-known of them is V. Frolkis’s adaptation and regulation theory that sees the formation of aging changes as interaction of two opposite processes, to-wit, aging and vitauct - a complex of processes inhibiting aging. Their interaction defines the specific syndromes and pecularities of the aging process, while any breaches in the balance boosts the development of age-related pathologies (70).
These are the common approaches to explaining the causes behind aging. However, apart from causes, there are also the conditions that favor or inhibit the development of this process. According to the modern understanding, multisystemic diseases in patients is what accelerates aging. A special role in the implementation of aging changes, to many authors’ belief, belongs to the condition of the cardiovascular, respiratory, nervous and endocrine systems, as well as disorders in lipid exchange and other kinds of metabolism (7, 16, 32, 63, 73, 150). The physical and chemical environmental factors promoting aging can include UV irradiation (104, 139, 157). It has been proven that eyes, skin and the immune system are especially vulnerable for UV rays (60, 62).
Currently, as state-of-the-art investigative methods are being developed, the process of aging is being actively studied at the molecular and submolecular levels. New evidence is being gained. Thus, the recent years have significantly expanded the knowledge about the role of regulatory proteins. Genetic theories are being streamlined, too. A. Olovnikov, the author of the telomerase theory, has suggested the “fountain” and redusome aging theories (43). An original idea of phenoptosis, developed by V. Skulachev (61), has been presented. Mathematical models of aging and mortality are also being employed actively.
Nevertheless, it can be said that during the last years there has been no breakthrough in gerontology with regard to theoretic understanding of the causes of aging. Therefore, it’s the well-known hypotheses that remain relevant, most of them set forth as long ago as in the 20th century. They gave start to many valuable approaches to healing aging.
1.3. Main principles of geropreventive therapy
The factors that slow down aging have been fairly well studied in our days and are still being studied. They are called geroprotectors (1, 17, 30).
One of the first classifications of geroprotectors, which we also believe to be the best suitable, was suggested by V.V. Zapadnyuk (1990) (23):
1. these are specific immune cytotoxic serums;
2. preparations of tissue therapy obtained through V.N. Filatov’s method;
3. adaptogens of plant, animal and synthetic nature;
4. combined preparations, like polyvitamins and aminoacid formulas;
5. antioxidants;
6. chelators (beta-aminocapronic acid);
7. neurotropic agents;
8. hormones;
9. anti-diabetes medicines;
10. amber acid, lipoic (thioctic) acid, etc/
Recently, these agents have been supplemented with immunomodulators and cell growth stimulators; preparations obtained from conoid glands (melatonin and epithalamin), as well as synthetic peptide bioregulators; extracts of embryoblasts; preparations preventing apoptosis; transcription and translation inhibitors; enterosorbents; antihypoxic drugs (17, 30, 56, 58, 73).
Lately, various rejuvenation technologies based on transplantation of autogenic or donor macrophages, as well as precursor cells of different tissues are being implemented (37, 77).
Diets (caloric restriction, protein-deficit), hypothermia, physical training, moderate stress, ionizing irradiation in small doses and other factors improving body resiliency are being used lately as geroprophylactic measures, in addition to negative electrostatic field and administration of microelements (selenium, chromium, potassium and magnesium, etc.) (1, 17, 34, 58).
1.4. Contemporary views of skin aging
Aging skin changes are a part of the entire body's aging process and obey the general biological laws of aging. However since skin is a barrier organ, it undergoes regressive changes faster than other organs do (62, 92).
In the last decade, various classifications have appeared for visual signs of skin aging (25, 82). These classifications are widely used in clinical practice.
Predominantly, there are three basic periods allocated in the process of aging changes:
- aging evolution: before age 20-25;
- aging changes stability: from 25-30 to 40-45;
- aging involution, starting from the age 40-45 (56).
Visual signs of aging, such as wrinkles, dryness and skin atrophy, show up at different ages in different people (102). The main factors accelerating skin aging are: excess insolation, smoking, cardiac and pulmonary diseases, excess body mass index (15).
Given the specificity of clinical changes in skin that appear under the influence of UV rays, scientists and clinicians have specified an additional aging type, skin photoaging. Not only does it have its own morphological and functional peculiarities but also requires special methods of prevention and correction (104, 129, 140).
1.4.1. Anatomic and morphological changes during skin aging
According to the American Academy of Dermatology, the morphological signs of skin aging include uneven pigmentation, yellowish skin tone, shaggy surface of the skin, wrinkles and folds, less elasticity and turgor, vascular malformations, and various neoplasiae (102). It's interesting how these changes gain momentum. A work by C. Guinot gives a detailed assessment of skin condition in 361 women aged between 18 and 80, according to 24 morphological positions (121). Before the age of 30, there were almost no signs of aging. After that, they began dashing up to the age of 71, where the curve reached another plateau.
Histological research confirm that signs of skin atrophy and distrophy show up after the age of 30. Aging change affects all its parts.
In the epidermis, there become less cellular lines (46). This is allegedly due to the decreased proliferative activity of ceratinocytes because of their decreased susceptibility to growth factors (25, 69, 132). These changes have been noticed in cases of skin aging over time (chronoaging). Where it comes to chronic solar exposure, ceratinocytes demonstrate high proliferative activity (152). Most researchers believe this is due to changes in the expression of several genes (163). Thus, where skin demonstrated signs of photoaging there were changes in the expression of genes related to the inhibition of the cellular cycle, DNA reparation, and protooncogenes (117). The damaging effect of UV irradiation over skin is mediated by large amounts of free radicals being formed in the skin (115). Not only do they damage the nuclear DNA by causing mutations, translocations, deletions, activation or inactivation of various genes, but also the mitochondrial DNA (1). The products of nuclear and mitochondrial DNA’s degradation are considered markers of chronological aging and photoaging of the skin. Mutations of mitochondrial DNA are a sign of chronic photo damage of the epidermis (87, 93). There is also information stating that resistance to ceratinocyte apoptosis is increased over age (95, 117). This further favors the accumulation of damaged cells in the epidermis. Histologic studies show perinuclear vacuolization of cytoplasm in ceratinocytes. In the basal layer cells, there appear to be low-prismatic cells and calcium salt conglomerates (60). The interdigital index is reduced (i.e. the ratio between the length of dermoepidermal junction in two points and the distance between them) (62).
Changes in the cell composition also affect melanocytes. There is an uneven distribution of them in the epidermis; the number of active cells is also reduced. The number of melanocytes grows significantly in photodamaged skin. However, they are smaller in size, have damaged nuclear structures, and contain products of melanin photodestruction. The amount of Langerhans cells is also decreased, and more significantly, in photodamaged skin, as well as their migration activity, which decreases skin’s resilience against pathogenic flora and increases the risk of neoplasms (62, 76, 96, 120).
Dystrophy and atrophy are seen still more clearly in the dermis. Its aging manifests itself in the gradual loss of the structure that used to serve as backbone. Over age, both the overall amount and the percentage of proliferating fibroblasts becomes lower (13). Lower rates of these cells’ renewal can, on the one side, be due to the decreased amount of mitosis-inducing proteins, and on the other one, because of accumulation of aging cells that are unsusceptible to proliferative and proapoptotic signals (6, 138, 143, 173). Since fibroblasts synthesize all the elements of the dermal extracellular matrix, including collagen, elastin, proteoglycans and minor proteins, their decreased amount and impaired functional condition result in the disbalance of synthesis and degradation processes in the dermis components. Also, the dermis becomes thinner, causing the most significant signs of skin aging to show up.
Both photoinduced and chronological aging causes decrease in the number of collagen fibers in the dermis, together with degradation of collagen, which is, however, more significant in photoaging (144). Morphometric studies of the skin of patients of various ages have revealed progressive decrease of collagen fiber contents, even in the areas not exposed to UVL. These changes manifest themselves by the age of 50 already and are especially significant in the medium part of the dermis (171). At the same time, there was a decrease in the contents of type 1 and 3 collagen and changes in the correlation between collagen of type 3 to type 1 (with the fraction of type 3 insoluble collagen prevailing). The degradation of collagen consists in the formation of collagen dimers. As a result, they become unavailable for the affect of matrix metalloproteinases (MMPs) and accumulate in the skin. Collagen with large amounts of seams (“seamed collagen”) is less elastic than normal collagen. It is also not good at binding water, which causes the dermis to dehydrate (40).
Over age, the quantity and quality of elastin fibers is also changed in skin. For various aging types, the changes have different directions: the amounts decrease at covered parts of the skin and increase at exposed ones. According to R.V. Korgunova, in women aged about 45 years old, with chronologic skin aging, and amount of elastin fibers per unit of area decreased threefold compared to the group of 25-35 year old women (33). During photoaging, the contents of elastin increased from 49.2% during the first decade of life to 75.2% during the ninth one. The increased production of elastin after exposure to UVL is believed to be because of increased expression of the genes responsible for its synthesis, under influence of UVL. The morphology of elastin and elastin fibers is changed. The processes of condensation, fragmentation, soaking, fiber dissociation also takes place, along with increased affinity to calcium salts (elastocalcinosis) and impaired orientation. In the papillary dermis, along with hyperelastosis foci, there are areas without elastic skeleton. Some authors believe this to be one of the principal markers of skin photoaging. In chronoaging, there is also degeneration of elastin fibers, especially noticeable in the reticular dermis (100).
The aging changes affect the extracellular matrix, decreasing the volume of basic matter, mostly proteoglycans, changing their qualitative composition, decreasing HA contents, increasing amounts of sulphated and neutral glycosaminoglicans and boosting their polymerization (111).
There are involutionary shifts in dermis microvessels. Over age, the number and sizes of vessels in papillary dermis are decreased, and their walls become atrophied (65, 100).
The main aging changes of skin appendages consist in their gradual atrophy. This relates to hair follicles, oil and sweat glands (100). According to L.D. Kalyuzhnaya, the number of eccrine sweat glands decreases about 15% during life. In general, the number and size of oil glands don’t change over age, but the production of sebum decreases by about 23% each 10 years (26).
1.4.2. Biochemical changes during skin aging
The physico-chemical and biological changes also affect all skin areas. Let us focus on the most significant ones.
Over age, the overall volume of skin lipids and the capacity to restore lipid layers after injury tend to decrease (97). This results in the decreased formation of the lipid-water film, which, in turn, increases water evaporation. This results in skin dehydration (45, 68).
Some researchers believe that overall water contents in physiologically aging skin increases. At the same time, there becomes less bound and more free water (18), which results in overall dehydration of skin together with swelling.
In 1963, F. Verzar provided rationalization for the collagen theory of aging, explaining the aging changes in collagen by the modification of collagen molecules through chemical reactions (165). Later, the reactions of nonenzymatic glycation were described. These processes are the cause of the disfunction of collagen-bearing structures in advanced age, not only developing in skin but also in kidney tissues, capillaries, cardiovascular and respiratory systems (64).
The biosynthesis of elastin grows over age, but skin elasticity decreases significantly at the same time. This apparent paradox is explained, on the one side, by intense disintegration of elastin as a result of proteolysis, and on the other one, by the disintegration of GAGs by enzymes and free radicals (46). With age, the activity of the most important proteinases increases. These include matrix metalloproteinase-1 and matrix metalloproteinase-13, responsible for the disintegration of native collagen, elastase, and serine proteinases. This decreases the contents of their inhibitors (tissue inhibitors of metalloproteinases, α-antiproteinase, α-2-macroglobulins) (100). The activity of the NO synthase enzyme is decreased, which results in impaired reaction of vessel myocytes to different stimuli (62). Interestingly, there is no consensus in literature regarding the condition of antioxidant defense (AOD) enzymes. Some authors believe that the activity of epidermal glutathione peroxidase, superoxide dismutase, peroxidase and catalase in skin extract is decreased, in the midst of unchanged activity of other enzymes (19, 156). Others denote moderate increase of peroxidase activity (135). It is shown that over age there is an increase of the contents of malonyldialdehyde and diene conjugates (19). It has also been shown that skin exudes water-soluble and lipophile low-molecular antioxidants whose activity and concentration decreases over age (90, 146, 151).
It has been demonstrated that fibronectin synthesis is increased, as is, though, also its proteolysis with age. Fibronectin fragments can have their own proteolytic activity and increase the formation of other proteolytic enzymes like collagenase (62).
A.N. Zimnitsky (2005) has suggested a concept of aging destabilization of GAGs as one of the biochemical mechanisms of aging. According to it, the genetically determined metabolism of intercellular matrix metabolism is impaired over age, which results in the depletion of GAGs from covering tissues and organs, while increasing their contents in blood serum. The author has demonstrated the substantial possibility of forecasting aging intensity through determining the fraction composition of GAGs in blood serum (24). The principal GAG-related aging changes in the dermis are the change of their qualitative composition and decreased amounts (106, 111). The decreased amounts of GAGs, primarily HA, impairs the processes of intercellular interactions. As a result, the gel structure of the dermis becomes more viscous, less permeable, and the dermis loses elasticity and turgor. The main causes for HA destruction is the effect of active oxygen radicals such as superoxyradicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen.
Once, much attention was paid to studying the role of “terminal toxin”, the very strong prooxidant lipofuscin, in skin aging processes. It is believed to be one of the markers of skin aging. Increased accumulation of lipofuscin in cells can be caused by hyperestrogenism, UV light, and stress (148).
1.4.3. Physiological changes in aging skin
Along with morphological and biochemical changes, aging skin also undergoes significant physiological transformation. It is generally believed that clinical and subclinical symptoms of involutionary skin changes are its decreased sensitivity, followed by impaired thermal regulation, significantly lower intensity of regeneration processes, chronical inflammations, impaired immunological reactions, signs of intoxication, particularly with FRO products (15).
It has been demonstrated that skin is closely related to the funcitonality of all the systems of the body: nervous, endocrine, immune, digestive, excretory, and musculoskeletal. Various problems in the body are manifested through skin. This is why some authors believe that the condition of the skin is an integral sign of the entire body’s aging rate. Interestingly, the first Russian method of determining biological age, suggested by P.N. Sokolova in 1935, was based on determining the skin wrinkling degree (15, 36).
It has been determined that skin aging is linked to significant changes in local neuroendocrine interactions. According to E.A. Yefimov (2000), I.O. Smirnova and I.M. Kvetnyi (2005), chronological aging of skin gradually suppresses the neuroendocrine activity of its cells. Meanwhile, in photoaging these changes are not uniform. On the one side, mast and endothelial cells are more likely to absorb and synthesize biologically active peptides and hormones; on the other side, the production of biogenic amines by keratinocytes is decreased in elderly patients (20, 62).
Over the last years, a connection has been discovered between chronic continuous local inflammation with involutionary skin changes, like the formation of winkles. This was called inflammaging (inflammation + aging) (118). It can be triggered by toxins, UVL, surfactants, and various stressors of other kinds. An important role in this process belongs to the cellular adhesion molecule ICAM-1. It is expressed at endotheliocyte walls and triggers a cascade of inflammatory reactions, first of all the activation of immune cells. The latter expresses large amounts of proteolytic enzymes that destroy collagen, elastin and free radicals, as well as damage the phospholipid membranes of cells and DNA. UV light also activates the NF- transcription factor, stimulating gene transcription for proinflammatory cytokines.
1.4.4. Principles of treating skin aging changes in aesthetic medicine
Aesthetic medicine is a part of medicine engaged in pathogenetic and symptopatic treatment of exterior defects of skin and its derivatives. Over the last years, the principles of aging changes treatment have been extensively revised, mostly because of accumulated knowledge of the differences between photo- and chronoaging. Due to this, the main principles of preventing age changes and mature skin care have been developed (39, 82, 164), including:
1. protection from damaging factors, firstly UVL and aggressive chemicals able to initiate LP (sun protection cosmetic products);
2. reconstruction and strengthening the barrier properties of the corneal layer (moisturizing cosmetic products, enzymatic peelings);
3. replenishing the local deficit of certain substances using external cosmetic products, mesotherapy, sonophoresis, ionophoresis;
4. blood circulation stimulation (manual and mechanical massage, mesotherapy);
5. stimulation cellular renewal of epidermis and corneal layer: surface peelings;
6. stimulation of dermal intercellular matrix renewal: mesotherapy, injections of platelet-enriched plasma, fractional photothermolysis, RF lifting, UV lifting, laser biorevitalization, injection of autologous fibroblasts (SPRS technology), medium-depth and deep peelings;
7. liquidation of aging symptoms:
- pigment stains: whitening cosmetic products, peelings;
- mimic wrinkles: botulinum toxin injections;
- skin contouring: wrinkle filling, UV lifting, RF lifting, plastic surgery.
Over the last years, these methods have been supplemented with other efficient measures. It has been shown that taking nutraceuticals with regulating peptides decreases the external signs of aging (8). E.V. Ivanova's work (2007) demonstrated how a course of subcutaneous injections of oxygen-ozone mixture decreased aging changes manifestation (improving skin structure and functional qualities, as well as microcirculation, thereby improving the overall index of clinical aging signs) (25).
1.5. Injection methods of treating aging change in aesthetic medicine
Modern cosmetic medicine employs a comprehensive approach to preventing and treating involutionary skin changes, including invasive and non-invasive methods, pathogenetic and symptomatic treatment. The most popular, effective and sought for among aesthetic methods are injection methods. These include microfiller augmentation, botulinum toxin injections, mesotherapy. It has been shown that correct prescription of methods taking into account the individual characteristics of a patient's skin aging, can postpone or completely avoid plastic surgery (12, 22, 27, 81).
1.5.1. Treatment of external aging signs using aesthetic injection methods
For the aesthetic correction of wrinkles, folds, scars and other small cosmetic defects of the “minus tissue” type, injections of microimplants (contouring agents, or fillers) have been used for the past 30 years. Augmentation is performed with preparations that can be classified into three groups by longevity:
- permanent, or long-term (over 5 years);
- prolonged (1.5-2 years);
- short-term (4-15 months).
The principal active ingredients of preparations can be natural (HA, collagen, autologous adipose tissue), synthetic (polyacrylamide gel), or complex (HA in combination with non-resorbable substances, like acrylic hydrogel). The injection depth for different drugs varies from dermal to periosteal (more viscous drugs are injected deeper). Histological studies have shown that fibrosis is normally formed at the injection site of any non-biodegradable preparations for contouring. Within 1-2 years, this is complicated by the formation of a capsule. This results in “plus-tissue” deformation. It should be noted that the fibrous capsule may eventually become dangerous and lead to complications (39), and, given the extensive commercialization of this field of medicine, the actual frequency of complications apparently exceeds the statistics. Biodegradable fillers do not have these shortcomings, however, their effect lasts no more than a year. Today's methods of using the patient's own collagen, adipose cells or fibroblasts are multi-stage, complicated, difficult to standardize, and therefore difficult to quantify, many drugs are still at the stage of clinical or preclinical trials and have not been implemented into practice (98). The most studied, physiological and safe ones today are HA-based fillers. However, even these hypoallergenic and non-toxic preparations can cause complications.
Aside from the properties of the filler itself, the body's response to its introduction is also affected by the characteristics of the patient, whether they comply correctly with the doctor's recommendation, and the correctness of the procedure itself - first of all, the depth of filler insertion. Complications caused by the injection of biodegradable fillers can be different. Necrosis of the nose bridge, edema, allergic reactions, herpetic infection can show up during the first days after the administration of the drug (101, 153). Later on, granulomas, rosacea, and secondary infection can develop (136, 149). The questions whether natural fillers, like stabilized HA, have a stimulating effect, and if a capsule forms around biodegradable implants, still remain open, and there are studies both confirming and refuting these effects (22, 80, 113, 168).
For more than 20 years, another injection method has been used, to-wit, injections of botulinum toxin (Clostridium botulinum exotoxin) to reduce overexpression of facial muscles and prevent the formation of wrinkles. The outstanding efficiency of the method, the quick onset of the effect and its prolonged duration have become the key to the widespread use of botulinum therapy: the number of procedures performed annually since 1995 has increased by about 700%, and this trend is likely to continue in the future. Different researchers who have studied complications in different years mention the same percentage of complications: 5-7%. Complications include allergic reactions, decreased sensitivity at the injection site, eyebrow and upper eyelid ptosis, periorbital edema, and diplopia [82].
Since even the most up-to-date preparations of botulinum toxin and microimplants provide a symptomatic effect only, the search for new preparations ensuring the same outside effect, but more long-term, caused by the activation of the internal reserves of the skin itself, is still relevant. In turn, the possibility of quite serious complications appearing during treatment of age-related skin changes with fillers and botulinum toxin makes us look for safer and more physiological methods.
1.5.2. Pathogenetic therapy of age-related skin changes with aesthetic injection methods
Pathogenetic methods include mesotherapeutic treatment of age-related changes. It is believed that when administered intradermally drugs are slowly diffused into the surrounding tissues from the injection site and gradually enter the circulatory and lymphatic systems, which can have both a local effect on the skin and a general positive effect on the body (42, 51). The pharmacological effect of the drug is enhanced by the puncture effect (from the very injection of the needle into the biologically active point or reflexogenic zone). It has been established that, in just a few minutes after the injection, a slight inflammatory reaction develops at the injection site, caused by the release of biologically active molecules (histamine, serotonin, NO, free radicals). It passes through all the main stages of inflammation: spasm and vasodilation, diapedesis of formed elements, release of the liquid fraction of the blood into intercellular space, and edema. Following this activation, fibrin accumulates in the dermis, the migration of formed elements accelerates, fibroblasts begin to produce growth factors, acid mucopolysaccharides, collagen and elastin fibers. Later, metabolic processes in the dermis improve, proliferation and apoptosis normalize, and microcirculation improves (4, 53, 78, 159). Given the above, mesotherapy can affect almost all pathogenetic mechanisms of skin aging. However, despite the widespread use of this technology, scientific studies on the efficiency of mesotherapy for the treatment of age-related skin changes are rather scarce, as are also studies on the efficiency of this technology, and their results are rather contradictory.
According to some authors, mesotherapy can be recognized as an effective means of correcting age-related changes in the skin and protecting it from the effects of harmful environmental factors (35, 159). A.P. Rozhanets (2007) demonstrates that using mesotherapy with Placenta Compositum and Ubichion Compositum preparations decreases the severity of wrinkles, improves skin elasticity and hydration, normalizes oil content in the facial skin, improves microcirculation and is more efficient in spastic-congestive type of microcirculation (55). V. Garcia et al. (2009) have studied the influence of intradermal injections of vitamin C and microelements (silicon, manganese, zinc) on turgor, microcirculation, severity of wrinkles, gravitational ptosis, and hyperpigmentation in female and male patients aged 40–60 years (101, 153). After 10 procedures, the integral indicator of facial skin aging, assessed on the Rubin scale, decreased in most patients, as did the severity of wrinkles, gravitational ptosis, and manifestations of hyperpigmentation (assessed on the MASI scale) (10). The authors concluded that the treatment was highly effective, however, as in most clinical studies of mesotherapy, the severity of involutory changes was assessed not instrumentally but subjectively, based on an external examination by a doctor, so this study was not double-blind randomized.
According to the results of other studies, there was no positive effect from mesotherapy on the severity of age-related skin changes, both in short-term and long-term observation (86, 89). A review by T.S. Tsai and V.M. Hantash (2008) analyzed the results of various studies on the use of mesotherapy for facial rejuvenation (162). The authors of this review note the large scatter of results, first of all. For example, a decrease in the severity of wrinkles after a course of transdermal injections of vitamin C, according to different authors, varies within 12–84%, and after injections of oligopeptides, within 13–84%. This may be due to the lack of a unified methodological approach, meaning difference in therapeutic courses, different duration of observations, and sometimes incorrect design of the study; in particular, most works give only a visual, rather than an instrumental assessment of clinical results.
A number of studies have shown that mesotherapy using placental preparations increases skin hydration and reduces the severity of wrinkles (130), and also increases the activity of fibroblasts (112). However, other authors believe that, to date, the possible negative consequences of mesotherapeutic administration of placenta extract have not been sufficiently studied. In particular, excessive activation of fibroblasts is possible (162). In recent years, cell therapy has been used to prevent age-related skin changes. Thus, it was shown that intradermal injection of keratinocyte and fibroblast cultures leads to revitalization of the skin and its appendages in experimental animals (77). The authors suggest that this effect is associated with the production of more collagen, elastin, enzymes, GAGs, and other biologically active substances by the transplanted cells, which can eventually improve the structure, hydration, and vascularization of the dermis. Clinical studies have shown that mesotherapeutic injection of fibroblasts from the human umbilical cord or autologous fibroblasts isolated from the patient's skin stimulates own fibroblasts to synthesize collagen, thereby increasing skin elasticity and its regenerative capabilities (83). However, despite no negative results after one year of observation, longer studies are required.
There are studies evaluating the effect of mesotherapy on the skin’s regenerative potential. For example, it has been shown that intradermal administration of ribomunil stimulates reparative regeneration of the skin (on a skin wound model in laboratory animals), improving the quality of a connective tissue scar formation in the skin, without significantly affecting the state of immunoreactivity of the body as a whole (3). T.G. Ruksha, V.V. Salamatin and I.A. Savchenko (2008) studied the intradermal administration of ascorbic acid to treat changes caused by UV exposure (57). Mesotherapy was proven to reduce spongiosis in comparison with a group of irradiated animals, while maintaining the physiological level of proliferation. These results may be an indirect evidence of the efficiency of transdermal BAS injections for the prevention of involutive manifestations.
Thus, the literature studying the use of mesotherapy to trat age-related skin changes is scarce and self-contradictory; these sources are far from covering all the drugs that are widely used to treat and prevent age-related skin changes.
1.6. Hyaluronic acid in anti-aging skin therapy
1.6.1. HA structure
Hyaluronic acid is a linear straight-chain negatively charged heteropolysaccharide, the main component of the extracellular matrix. GA is a nonsulfated glycosaminoglycan, consisting of repeating disaccharides N-acetylglucosamine and glucuronic acid, connected in turn by β-1,4- and β-1,3-glycosidic bonds (Fig. 1). A HA molecule can contain up to 25,000 of these disaccharide units. Natural HA has a molecular weight of 5,000 to 20,000,000 Da (134).
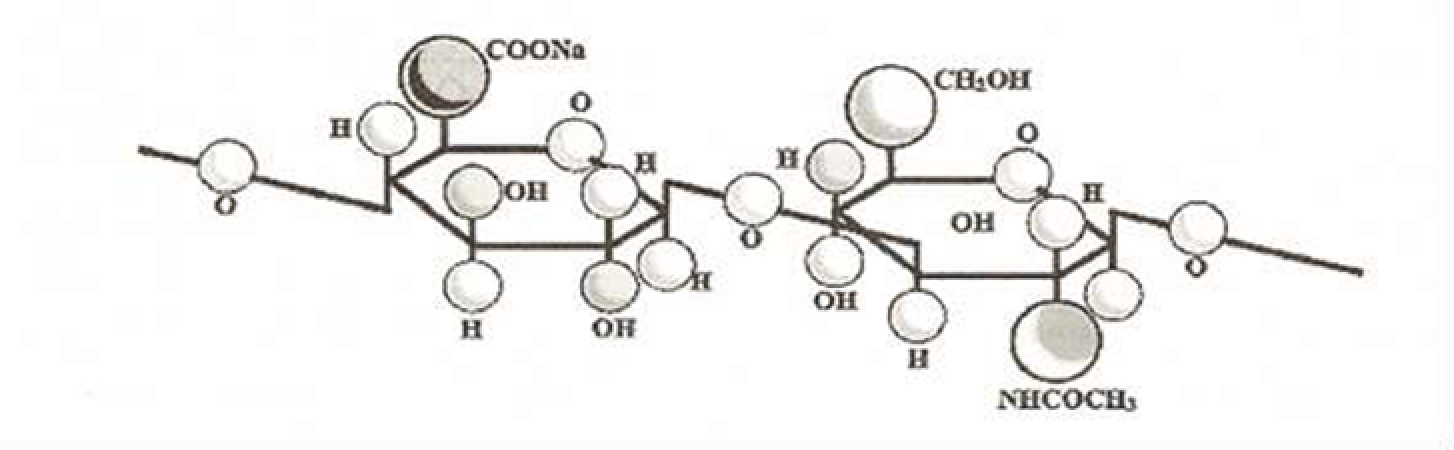
Fig. 1. Fragment of hyaluronic acid
The activity of biopolymers depends largely on the supramolecular structure. The secondary structure of HA is a spirally wound ribbon with a large internal space (due to a large bending angle). The tertiary structure is a loose coil of arbitrary shape (154). On the cell surface, hyaluronan exists in the form of many conformational types: linear chains, spirals, rod-like structures, “paper clips”. Through interacting with each other, HA chains form fibrils, stacks, nets. The supramolecular form of HA depends on its concentration in the solution, molecular weight, chemical environment, for example, whether it contains biopolymers that specifically bind to HA. As the polymer concentration in the solution increases, an intermolecular network is formed (52).
In the body, hyaluronic acid is present in the form of salts. High concentrations of it are found in some soft connective tissues, in the skin, umbilical cord, synovial fluid and vitreous body, in the lungs, kidneys, brain, and muscle tissues. About 50% of the body’s HA is found in skin, where it is located in the papillary layer among collagen and elastin fibers, along the vessels and appendages of the skin, and also in the granular layer of the epidermis. In the dermis, the amount of HA is 0.5 mg/g of tissue, and in the epidermis, 0.1 mg/g of tissue (72).
1.6.2. Main functions of HA
All the functions of HA in the body can be divided into non-specific and specific. Non-specific functions are associated with the state of intercellular substance, creating an optimal microenvironment for the normal functioning of cells, while specific ones have to do with the effect on cells through receptors. The structure of HA ensures its unique physicochemical and biological properties depending on the molecular weight and concentration of this biopolymer. Until recently, HA was considered as just a passive structural component of connective tissue, ensuring the biomechanical, trophic, barrier, and morphogenetic functions of the extracellular matrix. However, in recent years, HA has been spoken of as the most important regulatory molecule, and its mechanism of action is even compared with that of hormones and hormone-like substances. This wide spectrum of action explains the successful use of HA in various branches of medicine, including treatment of involutive skin changes.
Unlike proteins, HA never repeats its molecular form exactly. Due to this, its non-specialized functions are not affected even after some change in the structure of the molecule. And the main one of these functions is the supporting and structural one. Due to very high viscosity, HA, together with other GAGs, acts as cement binding collagen bundles and fibrils to each other and to cells. The space between collagen fibers is mainly occupied by HA. This neutralizes mechanical pressure on the skin. Physical pressure from the outside gradually “squeezes” HA from one space to another. Thereby, skin tissues become elastic against transient pressure and only gradually undergo deformation under the influence of constant pressure (59, 147).
Hyaluronic acid is a highly hydrophilic polymer. Due to the presence of negatively charged groups in HA structure, an HA molecule attracts a large amount of water (200-500). In the presence of water, HA molecules can increase in volume by a factor of 1000 and form a loosely packed hydrated matrix (67). Providing an optimal hydration degree of the intercellular matrix, HA creates physiological conditions for migration, division, differentiation of dermal cells, and normalization of all metabolic processes (94). HA also performs a barrier function by forming a "molecular" sieve preventing the flow of pathogenic agents (154). This ability of HA depends on the size and concentration of molecules. In the intercellular space, hyaluronate exists in the form of chains, and with an increase in its concentration, the chains form a three-dimensional network.
A dense network of chains is a fairly good obstacle to fluid flow and restricts the movement of pathogens, plasma proteins and proteases. The larger the molecule, the lower will be the speed of its movement through the hyaluronate solution. A sieve of hydrated hyaluronic acid polymers may be involved in the accumulation of physiological and pathological molecules in the connective tissue. By binding cations, HA also acts as cationic resin. Since all blood cations on their way to the cell must pass through this ion exchanger, this manifests the detoxifying role of HA, protecting the cell from exogenous and endogenous toxins. In addition, the polyanionic structure of HA is capable of capturing free radicals, thus possessing antioxidant properties and taking part in the regulation of inflammatory process. However, according to the studies of Khabarov et al. (2012), the antioxidant properties of HA are lower than those of classical AOCs (72).
HA’s physiological role in the body is also mediated by its specific interaction with cell receptors possessed by fibroblasts, macrophages, lymphocytes, and endotheliocytes. There are several types of hyaluron-binding proteins (hyaladherins):
1) proteins that bind HA to other extracellular matrix molecules, such as agrecan and verzican; by binding to them, HA regulates the organization of fibrin, fibronectin and collagen, cartilage tissue;
2) proteins that act as cellular receptors for HA. Two hyaladherins, CD44 and RHAMM (receptor of hyaluronic acid-mediated mobility), have been studied relatively well. CD44 (phagocytic glycoprotein Pgp-1, p85, ECMRIII, hermes antigen) is required for mediated signaling necessary for cell activation. The specific interaction of low molecular weight HA or its fragments with this receptor leads to the initiation of cell migration and stimulates cell proliferation. Binding of HA to the CD44 receptor can lead to a signaling cascade of activation of the synthesis of collagen, HA itself and other components of the intercellular substance, regulatory molecules in normal and pathological conditions (natural regeneration, repair of damage, maintenance of homeostasis, immunoregulatory, including anti-inflammatory reactions) (66, 88, 142).
Another hyaluronate-binding protein isolated later was hyaladherin RHAMM. The interactions between RHAMM and HA play an important role in the mechanisms of inflammation and tissue healing. RHAMM is involved in the regulation of cellular response to growth factors and cell migration, especially in fibroblasts (172). Literature also describes other signal transduction pathways triggered by HA through interaction with TLR, TNFIP6, LYVE-1, and SHAP receptors (126,172). Intracellular HA-binding proteins have also been described. They play an important role in cell cycle regulation and in gene transcription (161). Thus, the manifestation of HA’s physiological effects depends not only on its molecular weight but also on the level of expression of hyaluronan-binding proteins.
Speaking about biological activity, it is necessary to emphasize one unique feature of the “HA-receptor” system, namely, the importance of the molecular weight of hyaluronan. By controlling the course of such processes as inflammation, tissue repair, cell differentiation, morphogenesis and angiogenesis, HA macromolecules with different molecular weights have different effects on cellular behavior. Short-chain HA (400-10,000 Da) stimulates angiogenesis, cell migration and proliferation, while the HA fraction with a molecular weight of more than 500,000 Da inhibits angiogenesis and cell proliferation, and also blocks the synthesis of IL-1β and PG E2. The smallest fragments of HA (tetrasaccharides) inhibit apoptosis and stimulate the production of chaperones (heat shock proteins). Degradation products of hyaluronic acid with a size of 4–20 disaccharide units stimulate the growth of capillaries in vivo. In vitro, they induce endothelial proliferation, migration and the initial stages of vessel formation, and also have pro-inflammatory and immunostimulatory properties (110, 158, 166).
The HA fraction with a molecular weight of 50,000–100,000 Da has the ability to stimulate cell migration and proliferation and plays an important role in wound healing (107). It was found that on the first day of normal wound healing, an increased concentration of HA was observed. HA binds to the fibrin network, forming a transitional matrix that stimulates the activation of granulocytes, microphages, and fibroblasts (114). This improves the transfer of growth factors released from the cells improves, the migration of fibroblasts and the proliferation of epithelial cells (127). HA of low molecular weight, formed during the breakdown and rearrangement of the matrix, has an effect that enhances angiogenesis (170).
These differences are the basis for various uses of HA in medicine: the fraction with a molecular weight of less than 30,000 Da is recommended in case of risk of transplant rejection and in autoimmune diseases, since this fraction is an inhibitor of T-cell activity (137). High molecular weight HA with a molecular weight of more than 500,000 Da is used in cases where the formation of excess fibrous tissue and the formation of adhesions and rough scars are undesirable. The hyaluronan fraction with a molecular weight of 500,000–750,000 Da is a well-known chemotherapeutic agent that inhibits the proliferation of prostate, bladder, melanoma skin, and breast cancer cells (105). It has been shown experimentally that the efficiency of fibroblast protection from the cytotoxic action of free radicals grows with an increase in the size of HA macromolecule. Hyaluronan with a molecular mass of more than 1,000,000 Da is the most effective one in this respect (72).
1.6.3. Injectable preparations with HA for pathogenetic treatment of age-related skin changes
Currently, there are several methods of pathogenetic treatment of skin photo- and chronoaging using HA-based preparations. They use HA of animal origin, or biotechnological HA obtained from plant materials using cultures of non-pathogenic strains of bacteria Streptococcus zooepidermicus.
The first method is called biorevitalization. It was developed by the Italian scientist A. Di Pietro in 2001 and is interpreted as “a method of intradermal injections of unmodified hyaluronic acid, which makes it possible to restore the physiological environment and normalize metabolic processes in the dermis” (108). The method consists in making intradermal injections of native or modified HA with a molecular weight of 1,000,000–1,300,000 Da at a concentration of 8–25 mg/ml. The second method is the mesotherapeutic introduction of native HA as part of various cocktails. The concentration of HA in this case is 5 mg/mL or less on average, and the spread of molecular weight can be quite wide – from low molecular weight to high molecular weight fractions.
1.6.4. HA modification methods
Almost all GAGs of the human body are characterized by high metabolic rate: their half-life ranges from 3 to 10 days, therefore, in order to achieve the required duration of the HA effect, they are subjected to gentle stabilization. At the same time, the purpose is not only to change the structure of HA but also to preserve its biological compatibility and safety. Chemical stabilization (reticulation, modification) consists in chemical "cross-linking", i.e. the formation of crossed chemical bonds between linear HA molecules, with the formation of a three-dimensional network structure. At sufficiently high degrees of reticulation, the half-life of HA can range between 8 and 12 months (123).
To modify HA, various cross-linking agents are used, like carbodiimide derivatives, divalent metal salts, etc. In recent years, the use of diglycidyl ethers (DE) diols as cross-linking agents has attracted particular interest. This is due to the high biological inertness of these esters’ hydrolytic decomposition products, diols, especially ethylene glycol oligomers, including diethylene glycol. A method for cross-linking HA using DE diols in alkaline medium has been developed and patented. However, this method has some serious disadvantages: it requires using a large excess of reagents, making it difficult to predict the degree of cross-linking and purification of the reaction product; the cross-linking reaction proceeds along carboxyl groups with the formation of complex ether bonds, which are rapidly broken (160).
There is a more advanced method of HA cross-linking, where the reaction between HA and DE diols occurs in an acidic medium, so the cross-linking proceeds along the hydroxyl groups of HA with the formation of simple ether bonds, which are slowly destroyed in the body. The acidic medium is created by adding hydrochloric acid. A method has been developed and patented comprising an additional stage of partial HA deacetylation and subsequent cross-linking using toxic aldehydes and isocyanates.
In addition to chemical methods of HA cross-linking, there is also a photochemical method for cross-linking modified HA by means of UV radiation exposure (5). However, using this method is very difficult, since it means the introduction of an additional laborious stage of HA modification with derivatives of cinnamic acid or carbodiimide (123).
A fundamentally new method of HA modification has been patented recently. This is solid-phase HA modification with bioactive compounds (vitamins, amino acids, oligopeptides). Solid-state synthesis doesn't employ acids and alkalis as solvents, thereby making biopolymer modification processes environmentally cleaner. The polysaccharide modification with various bioactive compounds occurs simultaneously with the mixing of the two main components in solid state, using increased pressure and shear deformation, which ensures not only their alignment at the nanoscale level but also the occurrence of various chemical interactions in the absence of a liquid dispersion medium. This makes it possible to graft water-insoluble compounds (folic acid, retinol) onto the HA molecule, and not to change their spatial structure when working with amino acids and peptides (71). When using substances with high geroprotective, particularly antioxidant, effect to modify HA, it is possible to create unique combinations of ingredients with predetermined useful properties. This way of creating drugs is very promising, but there is still not enough experimental and clinical evidence of their high efficiency and safety in the literature, as well as no results of comparative studies for various drug types based on modified and unmodified HA.
1.6.5. Results of involutive skin changes treatment with injectable HA-based preparations
According to a 2009 report of the American Society for Aesthetic and Plastic Surgery (ASAPS), hyaluronic acid (HA) injections take second place among the other non-invasive methods of skin rejuvenation (1,313,038 HA injections in the USA, 2009), ranking next to botulotoxinum A injections only (2,557,068 injections in the USA, 2009) (125). Along with the growth of clinical use, the number of scientific publications on the effect of HA-containing drugs on age-related skin changes is also growing.
From the works dedicated to the results of involutional skin changes treatment with HA, publications by S.V. Moskvin et al. should be singled out. This group analyzed the results of introducing various HA gels by means of laser phoresis and low-intensity laser radiation into the skin of patients of different age groups (40). The authors give clinical evidence of this therapy’s efficiency, show how the results depend on the drugs’ physicochemical characteristics and methods of administration. However, they do not compare non-injection administration of HA with injections, which would be interesting since laser phoresis administration does not provide complete intake of injected drugs into the dermis.
A thesis by N.S. Zhirnova (2007) studies the reaction of the surrounding tissues to the introduction of filler gels based on stabilized HA (22). It has been shown that HA preparations affect the mechanisms of skin structures regulation and immune processes in the area of their administration. The efficiency of stabilized Ha-based fillers has been proven in relation to the correction of wrinkles, atrophic scars, and the elimination of age-related ptosis of face and neck soft tissues, as well as changing the shape of the lips. Based on histological data, the author concluded that there was an increase in the mass and an improvement in the structure of collagen and elastin fibers, explaining this by the fact that the injection of HA favors the increase in the fibroblasts’ mitotic activity, however, no quantitative indicators confirming this are given in the work. As a practical recommendation, it was noted that during the first 10 days after the administration of the drug one should avoid effects that can aggravate the already existing edema, hyperemia and increased permeability of the vessel walls, and also not administer stabilized HA to persons with aggravated allergies.
An article by Gensanne D., Josse G., Schmitt AM. (2007) presents the results of morphological skin studies after injections of stabilized HA, however the main task of the researchers was to visualize the Ha-based gel and the onset of its degradation time (116). A similar study examining the distribution of various augmentation gels was carried out by T.S. Flynn et al. (2011). They demonstrated uniform distribution of drugs in the dermis, and that in different types of gels the distribution in tissues was different. No complications were recorded (113).
Baspevras M. et al. (2013) described positive clinical results of the introduction of preparations based on unmodified HA into the skin in order to correct involutional changes, but there were no comparisons of clinical data with the results of morphological studies (91).
Alessandrini A. et al. (2006) studied the residence time of 1% and 2% stabilized HA gels and native high molecular weight HA in tissues. Histological studies of the skin of experimental animals did not reveal any signs of inflammation, tissue degeneration or necrosis for all studied preparations and showed a prolonged presence of 2% HA gel in tissues. However, the experiment revealed no structural changes in the skin confirming the positive effect of drugs on age-related skin changes (85).
There is some literature showing the effectiveness of intradermal administration of HA for the treatment of actinic keratosis, however the authors did not study the effect of HA on other external signs of age-related skin involution (169).
According to M. Kerscher et al. (2008), administration of stabilized HA at a concentration of 20 mg/mL (NASHA) improved skin elasticity and microrelief (128). Similar results in the long term after several procedures of HA injections into the facial area were obtained by other researchers who noted a significant increase in skin density and elastic properties with the introduction of both native and stabilized HA (79, 133).
An ultrasound study of the efficiency of a 4-week course of a HA product microinjections for biorevitalization showed a significant decrease in the severity of UV radiation damaging effect on hand skin cells in 15 out of 20 women included in the study (age 40–60 years) (131).
Along with studies that have revealed a positive effect of HA injections on the structure and functional parameters of the skin in patients of middle and older age groups, literature also mentions the absence of any changes after intradermal administration of HA. Amin S.P. et al.(2006) assessed the histological and clinical changes in hands skin after a course of rejuvenating intradermal injections of multivitamins and HA. Based on the results, the authors concluded that the use of multivitamin complexes and HA solution in mesotherapy did not bring significant benefits, since they did not find any significant clinical and histological changes (86). A work by Shishkova I.V. (2000) showed that intradermal administration of HA after local thermal damage to the skin did not affect the activity of blood mononuclear cells, as well as the immune reactivity of the skin, in particular, the development of humoral immune response and delayed-type hypersensitivity (80). The author did not specify the characteristics of the HA.
Thus, of the available literary sources, there are practically no works offering both experimental and clinical studies. The descriptions of research methods do not always give a complete description of the drugs used, their molecular weight, molecular weight distribution, concentration, and method of HA modification. Quite often there are methodological errors, subjective evaluation of treatment results, and imperfections in the statistical part. This makes it difficult to compare and interpret the results. In the available literature we only found a few studies giving a comprehensive assessment of HA preparations' effects. A work by Wang F. et al. (2007) studied the effects of stabilized HA for injection augmentation to treat photoaging signs. The study included histochemical analysis and optical microscopy, PCR to study the expression of type I and III collagen genes, genes encoding connective tissue growth factor and transforming growth factor, matrix metalloproteinases and their inhibitors. With regard to preparations for augmentation based on modified HA, the authors proved the effect of clinically significant stimulation of collagen synthesis in the dermis, which they explained by an increase in the synthetic activity of fibroblasts and the development of neocollagenogenesis in the area of drug administration, as well as weakening the processes of collagen destruction as a result of increasing amounts of matrix metalloproteinases inhibitors (167).
Evaluating the results of studies on HA use for skin rejuvenation in aesthetic medicine, it can be noted that the accumulated clinical experience is far ahead of scientific research. There is little data in the literature concerning the effects of exogenous HA introduced into the dermis, its use in the therapy of photo- and chronoaging, and the effect of exogenous HA on the mechanisms of neocollagenogenesis. There is a large number of conflicting facts and issues that need to be studied. Multicenter, double-blind, randomized, placebo-controlled studies of intradermal administration of various forms of HA are required. As clinical practice is enriched with new, more advanced HA preparations and technologies for their manufacture based on modern information about HA, they also require advanced studies. In particular, preparations of HA stabilized by solid-phase modification, which include biologically active substances covalently bound to HA and traditionally used as geroprotectors, have not yet been sufficiently studied. Meanwhile, an experimental and clinical study of such drugs' action would not only allow us to propose new effective ways to correct age-related skin changes, improve the quality of life of the elderly and increase the efficiency of dermatological diseases treatment, but also expand the theoretical understanding of skin aging and the role of HA in this process.
2.1. General characteristics of the experimental study
Experimental studies were performed to study the characteristics of resorption (rate, completeness, tissue reaction) of hyaluronic acid gels after subcutaneous (series I) and intradermal (series II) administration.
1st series of experiments: subcutaneous administration of HA gels. In the first series of experiments, 5 options of hyaluronic acid gels of various degrees of structuring were studied, containing various inclusions: vitamins, amino acids, oligopeptides, excipients.
1. Native (unmodified) HA hydrogel (Gel No. 1)
2. HA hydrogel modified with vitamin C (Gel No. 2)
3. HA hydrogel modified with glutation (Gel No. 3)
4. HA hydrogel modified with folic acid (Gel No. 4)
5. HA hydrogel modified with vitamin C, proline, glycine, lysine (Gel No. 5) HA content in the gels was 1.4 wt.% in all cases, the content of additives was 0.4 wt.%
The experiment was performed on 180 white laboratory male rats weighing 120–140 g, obtained from the nursery of experimental animals of the Scientific Center for Biomedical Technologies of the Russian Academy of Medical Sciences (Andreevka branch). In accordance with the 1986 European Convention for the Protection of Experimental Animals, the animals were kept under standard vivarium conditions, 3 individuals per cage, fed with complex granular laboratory food with constant access to water.
Animals were anesthetized by intramuscular injection of Zoletil solution (Zoletil 100, Virbac S.A., Italia) at the rate of 6 mg of active ingredient per kg of body weight of the animal, in combination with rometar (Rometar, Spofa, Praha) at the rate of 0.5 ml per kg of body weight. Anesthetized animals were injected with 1.0 ml of the studied gel samples in the interscapular region of the back to the left of the midline, subcutaneously with a sterile needle. On the right, a 5% solution of native hyaluronic acid was similarly injected (Fig. 2).
Each group included 12 animals.
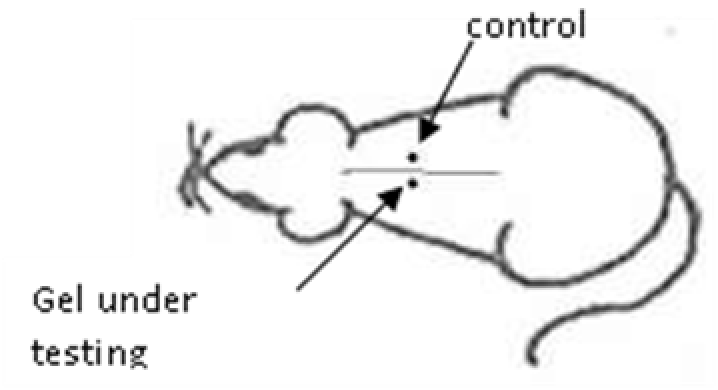
Fig. 2. Scheme of administration of HA gels to experimental animals
Animals were taken out of the experiment within 1, 3, 7, and 14 days. A macroscopic visual assessment was performed of the gels injection sites, checking their safety and appearance, the presence of a capsule, the condition of the surrounding tissues, etc. After that, the remains of the gel together with the capsule were taken for subsequent histological study. The selected material was fixed in 10% neutral (buffered) formalin solution. After 3 days of fixation, a sample of 0.5–0.8 cm in size was cut out from the biopsy and embedded in paraffin blocks according to the standard method. Paraffin sections of 4-5 microns were stained with hematoxylin and eosin, Van Gieson's picrofuxin (to reveal mature collagen fibers), and toluidine blue for acid glycosaminoglycans (GAGs). In some cases, the Gomori silver stain was also used to identify collagen fibers of various degrees of maturity and the Brachet stain for RNA was performed. The resulting micropreparations were studied using an Olympus BX51 light microscope (Olympus, Japan) equipped with an SDU-252 digital video camera (Spetsteletechnika, Russia). Microphotography of histological preparations was carried out using this camera and the Launch Cam_View software. The resulting images were saved in Tiff (16 bit) format.
2nd series of experiments: intradermal administration of HA gels.
In this series of experiments, resorption and tissue reaction were studied during intradermal administration of hyaluronic acid gels. The experiment was carried out at the Center for Biomedical Technologies of the Russian Academy of Medical Sciences on 3 minipigs of the Svetlogorsk breed, weighing 16-20 kg.
3 options of hyaluronic acid gels were studied:
1. Native hyaluronic acid (control)
2. HA hydrogel modified with vitamin C, glycine, proline, and lysine
3. HA hydrogel modified with vitamin C and glutation.
The gels were injected intradermally into depilated areas of the posterolateral surface of the back through 3 injections 1.5 cm apart of each other. The injection sites were highlighted with a marker.
Observation terms: 1, 3, 5 days. At the specified time, a strip of skin was excised under local anesthesia in the area of gel injection. The resulting wound was sutured tightly. From the entire thickness of the skin at the injection sites (through the center of the injection), 2 samples of 5x8 mm in size were cut out for histological and electron microscopic examination. Sample fixation, sectioning and staining for histological examination were performed in the regular manner (described above).
Electronic microscopic examination of biopsy specimens taken from all 3 animals was performed on the 3rd day after the administration of HA gels.
To prepare for transmission electron microscopy, the excised skin samples were kept for 10–15 min in Ito fixing solution, following which fragments of 2.0 × 1.0 × 1.0 mm in size were cut out from them. The selected material was further fixed in Ito solution at 40С for 2 days, after which the samples were washed with a 0.1 M solution of cacodylate buffer (pH 7.2–7.4) and additionally fixed in 1% solution of osmium tetroxide in 0.2 M cacodylate buffer at the same pH for 1 hour. At the end of fixation, the samples were dehydrated in ethanol solutions of increasing concentrations, then they were transferred into absolute acetone and poured into a 1:2 mixture of epon-araldite resins. After polymerization, semi-thin sections 1–1.5 µm thick were made from blocks of tissue embedded in resin and were stained with toluidine blue. Semi-thin sections were examined with a light microscope in order to select the tissue area required for electron microscopy. After targeting the desired tissue area on an LKB ultramicrotome (Sweden), ultrathin sections (25 nm thick) were made and placed on blends. Sections were counterstained with saturated uranyl acetate in methanol and 0.4% aqueous lead citrate. The preparations were studied using a JEOL JEM-2000 FXII transmission electron microscope (Japan) at an accelerating voltage of 200 kV and special Digital Micrograph Ink software (JEOL, Japan). The resulting images were converted to the universal TIF graphic format.
When studying skin ultrastructure at the sites of HA gels injection, the relative electron density of visible objects and the sizes, functional states, relative positions and orientations of cellular and fibrous elements were determined. The presence or absence of pathological change manifestations in skin structure were also noted.
2.2. General characteristics of the examined patients
The clinical part of the study involved 92 mature (from 35 to 50 years old) and elderly (from 60 years old) female patients. At the time of the study (for 1.5 months), all patients kept to a normal work and rest regimen, a typical diet, and did not experience stress. The study did not include patients with severe diseases (organ and system failure, diseases in the exacerbation phase), who had suffered severe injuries or undergone surgical interventions. Women who were on diets, flew airplanes, were stressed during therapy, or discontinued treatment were excluded from the study
All patients had age-related changes in their skin, as well as manifestations of photoaging – decrease in skin elasticity, wrinkles and folds (dynamic mimic and static-mimic), uneven complexion, as well as dehydration signs (from a feeling of tightness after contact with water to the presence of exfoliation during the day, uncompensated or compensated to a small extent by the use of moisturizing creams). In groups 2 and 3, the patients had pigmented disorders in the form of sun-induced lentigo.
At the start of the study, none of the patients had previously used any anti-aging injection techniques. For home care, it was recommended to use healing agents (Traumeel cream) for 5-7 days after the procedures, and then (until the next procedure) to use moisturizing creams.
Pain relief measures were taken based on the empirical assessment of the patients’ pain threshold. Application anesthesia was used with 5% Emla ointment for 15-20 minutes before the mesotherapy session. Of the 20 patients, this method of anesthesia was used in 9 women, the rest of them received the drug without anesthesia.
The mean age of the patients participating in the study was 47.4 years (minimum age 35 years, maximum age 62 years). The patients were divided into 3 groups according to the severity of manifestations of photo- and chronoaging (according to the Glogau scale – Table 1):
1 group – presence of 2-3 signs of type II – age from 35 to 40 years;
2 group – prevailing of signs of type II – age from 41 to 55 years;
3 group – prevailing signs of type III – age from 56 to 62 years.
Table 1
R. Glogau photoaging scale (119).
Type I
• No or minimal wrinkles
• Subtle symptoms of photoaging
• No pigmentation
• No keratomas
Type II
• Emerging mimic wrinkles
• Mimic wrinkles become noticeable when facial expressions change
• Mild to moderate symptoms of photoaging
• Early senile lentigo
• Keratomas are felt when touched, but visually they are still hardly noticeable
Type III
• Wrinkles are noticeable even with a calm facial expression
• Significant symptoms of photoaging
• Hyperpigmentation and telangiectasias
• Keratomas and other benign neoplasms
Type IV
• Numerous deep wrinkles are visible
• Excessive symptoms of photoaging
• Yellowish-gray complexion, numerous age spots
• Numerous keratomas
• Often – neoplasms, including malignant ones
The distribution of patients by groups is shown in Table 2.
Table 2. Age breakdown of patients selected to participate in the clinical study
Study groups
Group 1
Group 2
Group 3
Number of patients
30
31
29
Patients' ages, years
35 - 40
41 - 55
56 - 62
Average ages of the patients, M±m
36.4 ± 0.90
48.3 ± 0.62
60.97 ± 0.67
2.3. Non-invasive methods for assessing morphological and functional parameters of patients' skin
To assess the dynamics of the morphological and functional parameters of the patients' skin, non-invasive methods for diagnosing skin functions and morphology were used (Table 3).
Table 3. Brief classification of non-invasive skin diagnostics methods
Skin functions diagnosing methods
Skin morphology diagnosing methods
1. Moisture measurement – corneometry
2. Skin pH measurement – pH-metry
3. Sebum secretion (fat content) assessment – sebometry
4. Skin elasticity assessment – elastometry
1. Skin microtopography assessment – ultrasonic dermascanning
2. In vivo study of skin morphology – confocal laser scanning microscopy
3. Evaluation of skin relief – visual scanning
The measurements were taken before the start of the treatment (initial skin condition) and 3-4 weeks after the completion of the procedures.
2.3.1. Ultrasonic dermascanning
For non-invasive assessment of skin microtopography in patients with involutive skin changes before and after the administration of modified HA preparations, the ultrasonic scanning method was used using the DermaScan C ver.3 device (Cortex Technology, Denmark). Ultrasonic scanning is a high-resolution method of skin structure visualization, making it possible to observe the skin structure up to 10 mm deep and to determine the thickness of the epidermis and dermis, their acoustic density and other parameters.
The complete set of the device includes:
1. Monitor
2. System unit
3. Ultrasound scanner
4. Special sound-conducting gel
5. Roll of polyethylene membrane
6. Distilled water dispenser
7. Ring to fix the polyethylene membrane
The DermaScan C ver. 3 ultrasound scanner generates a mechanical wave with a frequency of 20 MHz. The wave penetration speed depends on the density and elasticity of the medium, and the resulting acoustic impedance (ultrasonic vibrations) is the result of a change in the wave propagation speed in the tissue. The difference in acoustic impedance in the surrounding media determines the echogenicity. The source of ultrasound sends a signal in pulsed mode. During the pause, the receiver waits for the reflected wave. The image on the monitor screen reflects the dependence of the electrical voltage corresponding to the ultrasonic signal sent and recorded after reflection.
For the study, two modes of skin scanning were used, A and B.
In scanning mode A, a narrow directional ultrasound signal passes through the tissue in order to obtain an image along a certain axis. In this case, the distances between objects and tissue structures are measured horizontally (in depth), vertically and arbitrarily. The measurement results are displayed in the form of a graphic image created using the device's computer software.
In scan mode B, a vibrating ultrasound signal passes through the tissue in order to obtain an image in a certain plane. The mode allows setting the thickness of structures by automatically detecting boundaries and dividing the image into segments depending on color intensity, followed by image analysis.
Water was used as a conductor of the high-frequency ultrasonic wave in order to minimize sound signal attenuation. Therefore, there is an internal water chamber inside the scanning heads and a polyethylene membrane is introduced to prevent water leakage. The membrane is made of special polyethylene film that transmits ultrasonic signal, minimizing scattering and resulting in high image quality.
The change in the speed of sound during scanning is used to calculate distances. Different tissues have different densities, and the speed of sound will always be different in them. In human tissues, the speed of sound propagation depends on their structure. The most commonly used figures are as follows: skin – 1580 m/s, fatty tissues – 1450 m/s, nails – 2470 m/s. The value of 1580 m/s is set in the device by default, since this is the speed of sound for most areas of the skin. During the study, an assessment was made for the state of the epidermis (thickness, uniformity, discontinuity of this layer) and the dermis (quality of the subepidermal layer, uniformity and size of the structures of this zone).
2.3.2. Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) was carried out on a Vivascope 1500 device (Lucid, Inc., USA). This method makes it possible to obtain images of cells in real time, layer by layer, on both living and excised tissue, and it approaches optical microscopy in terms of resolution.
A diode laser with a wavelength of 830 nm and a power of not more than 28 mW on the skin surface is used as light source in the microscope. To create an immersion medium between the lens and the adhesive window, a dense ultrasound gel (Sonogel, Vertriebs, Germany) was used, and a special cosmetic oil (Contacting agent STS, Mavig, Germany) with an optimal refractive index was used between the window and the tissue. The device allows in vivo skin scanning to a depth of 250 µm. The size of each received image on the screen is 500x500 microns. The scanning was carried out using a 30x immersion objective with a focal length of 5.3 mm.
Layer-by-layer skin study was carried out by obtaining optical sections along the Z axis of the microscope step by step to a depth of 2-5 μm from the surface of the stratum corneum (optical "zero") to the first cells of the granular layer with large nuclei, then deep down to the tops of the dermal papillae, to the base of the papillae and the collagen fibers layer.
2.3.3. Study of skin microrelief
The Visioscan VC 98 device (Courage+Khazaka electronic, Germany) was used to assess the indices of skin microrelief in the process of selective pulse exposure.
The Visioscan video camera consists of a high resolution video sensor and a UV-A light source. The video sensor reproduces a black and white image of the skin with 256 shades of gray. These shades are used to calculate such parameters as unevenness, roughness, and wrinkling of the skin. The built-in ring-shaped UV-A light source evenly illuminates the skin and is completely harmless to it. A special source emits light mainly at a wavelength of 375 nm, eliminating unwanted reflections from the skin and providing a clear, contrasting image of skin and hair. The spectrum, intensity and illumination angle of the light are selected in such a way as to monitor the stratum corneum without reflections from deeper layers of the skin. The measurement area is 6x8 mm.
Visioscan is connected to an IBM compatible PC and a video monitor. The Visioscan device was connected to a PC using a digital unit that configured the image according to 256 gray levels from pixel to pixel, where 0 was black and 256 was white. The SELS (Surface Evaluation of the Living Skin) software, developed for Windows at the Institute for Experimental Dermatology at the University of Witten-Herdecke, Germany, analyzed the image, calculating and evaluating the geometric characteristics of skin surface. The software generated a 3D digital color image of the skin surface, a 2D surface profile plot and a histogram (graphic representation of the distribution of colors in an image from light to dark).
The procedure for operating Visioscan VC 98 was as follows: using a video camera, a skin area was recorded on a video monitor, after which the image was displayed on a PC screen. The SELS program was used to digitally process the image displaying numerical information about skin surface microrelief on the screen. The study was conducted at standardized points.
2.3.4. Study of the skin`s elastic capacity
The study was carried out using the nozzle of the Soft Plus device (Callegary, Italy) at a standardized point according to the protocol for measuring skin elasticity on this device – the “crow's feet” area (Fig. 3).
Fig. 3. Soft Plus elastometry scale (Very low; Low; Normal)
The Cutometer MPA 580 includes a gauge head with aperture and negative pressure tube and a digital unit that is connected to an IBM compatible PC.
The principle of its operation is based on the photometric determination of the rate of skin area deformation and the degree of restoration of the original shape under the effect of vacuum. The deformation is induced by applying negative pressure to the skin in the range of 20 to 500 mbar. The skin area to which negative pressure is applied is retracted into the measuring head’s aperture. The depth of skin retraction into the aperture is determined by optical sensors built into the head. Mechanical pressure on the skin is controlled by a special spring in the measuring head. The device's electronic circuit compensates for the temperature effect on the measurement results. The measurement results are processed using special software for Windows.
The measured parameters were displayed graphically in the Windows environment. The duration of measurement cycles can vary from 0.1 to 60 sec.
The operation procedure was as follows: the measuring head was applied to the skin area; then, after pressing the OK button, negative pressure was applied to the measuring head tube and continued in the specified mode. The measurements were displayed graphically on a PC monitor. At the end of the measurements, the software processed the results returning the parameters characterizing the skin`s mechanical properties.
2.3.5. Study of skin moisture
The study was carried out using the nozzle of the Soft Plus device (Callegary, Italy) at standardized points according to the protocol for measuring skin moisture on this device – central part of forehead, central part of both cheekbones, chin (Fig. 4).
Fig. 4. Soft Plus corneometry scale
(Norms Skin moisture very low Skin moisture below normal Normal skin)
The method makes it possible to determine the water content in the water-lipid mantle of the epidermis’ upper layers using the capacitance method, based on differences in the dielectric constant of water (81) and other substances (usually < 7). The measuring capacitor shows changes in electric capacitance corresponding to the water content.
Procedure of measuring humidity:
For all measurements, the optimal conditions were observed: temperature of 20°C and relative humidity of 40-60%. To measure moisture, a measuring head was applied to an area of the skin, in accordance with the pressure of the spring in the head. The device started measuring immediately after contact with the skin. The subsequent signal meant that the measurements were complete. The “H” sign and the measured value appeared on the display.
The results were compared with normal values in the range from 0-130 arb. units, which correspond to the indicators in the case of normal skin under normal environmental conditions at a temperature of 20°C and air humidity of 40-60% (Table 4)
Table 4
Interpretation of moisture measurement results
Forehead, t-zone, scalp, corners of the mouth, upper body, back, neck
Arms, hands, legs, elbows
Very dry
less than 50
less than 35
Dry
50-60
35-50
Moist enough
more than 60
more than 50
2.3.6. Skin pH study
The study was carried out using a special nozzle of the Soft Plus apparatus (Callegary, Italy) at a point corresponding to the center of the zygomatic region (according to the pH-metry protocol on this device, Fig. 5).
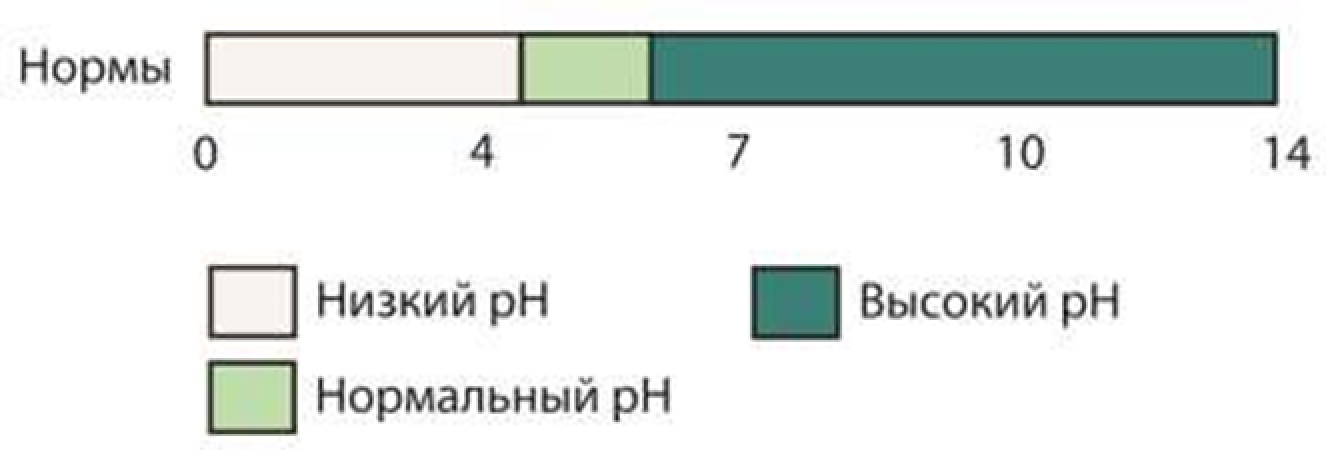
Fig. 5. Soft Plus pH-metry scale (Norms Low pH Normal pH High pH)
The pH level was measured using glass electrodes, which were filled with an internal buffer (silver chloride Ag/Ag CL). This internal buffer solution is separated from the measured solution with a special glass membrane and diverts the potential from the inside of the glass membrane (through the metal contained in the solution). The main electrode is filled with electrolyte and equipped with a diaphragm that guarantees the transfer of ions between the measured solution and the internal buffer solution, but prevents mixing of these substances.
pH measure procedure:
1. Before the start of the measurement, the measuring head was rinsed in distilled water.
2. Turning on the device.
3. The measuring head was applied vertically to the skin for 3 seconds (at this time, the clock on the display started counting down).
4. A signal after 3 seconds meant that the measurements were completed, and the measured value was displayed on the display.
The results obtained were compared with the following values, which are observed in the study of normal skin under normal environmental conditions: at a temperature of 20°C and air humidity of 40-60% (Table 5)
Table 5. Interpretation of pH-metry results
Gender
Sex
<3.5
3.8
4.0
4.3
4.5
5.0
5.3
5.5
5.7
5.9
6.2
6.5
>6.5
Female
acidic range
normal
alkaline range
Male
acidic range
normal
alkaline range
2.3.7. Study of the sebaceous glands' function
The study was carried out using the nozzle of the Soft Plus device (Callegary, Italy) at a standardized point according to the protocol for sebometry on this device – the central part of the zygomatic region (Fig. 6).
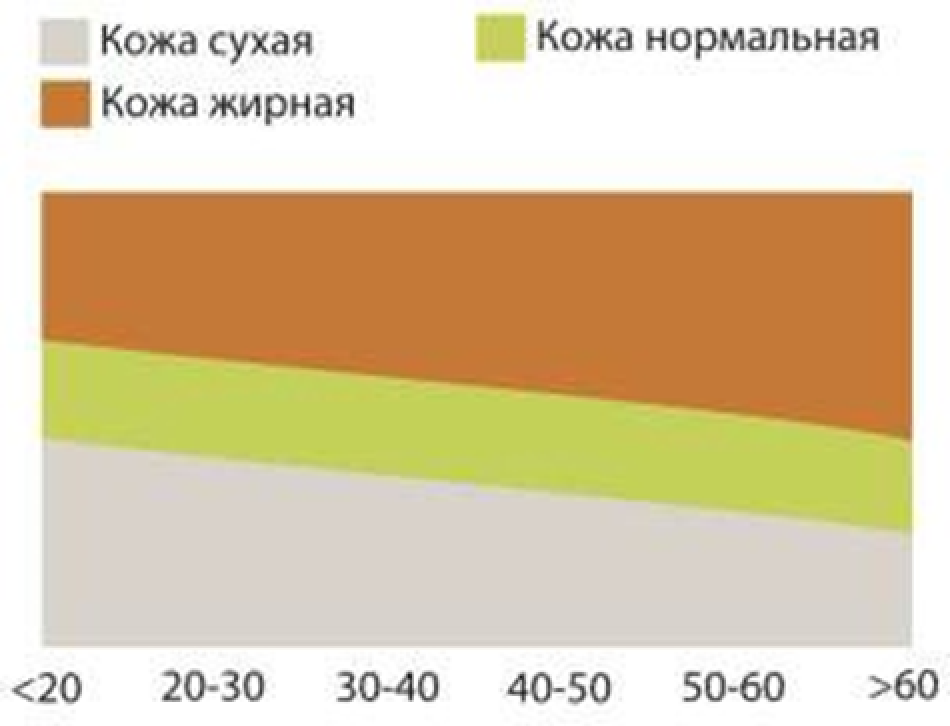
Fig. 6. Soft Plus sebometry scale (Dry skin Normal skin Oily skin)
The assessment of sebum secretion (sebometry) allows you to determine the amount of sebum lipids on the surface of the face and scalp skin through photometry. During testing, a special adsorbing tape impregnates the skin secret. The tape is placed in a photometric sensor, in which the photocell measures the degree of light penetration through the polymer, depending on the amount of sebum adsorbed on it. A microprocessor uses a special program to recalculate the measurement results and output the final data in µg/cm2. This method is selective, the measurement results are not affected by the degree of skin moisture.
The device has 2 phases – zero and measuring. Switching between these phases is carried out automatically when the device's cassette is inserted into the appropriate aperture. The cassette was inserted into the aperture and pressed for 1 second until the signal for the indication to appear on the display.
To measure greasiness, a measuring cassette was applied to a skin area. After measuring for 7 seconds, the cassette was inserted back into the aperture and the value from the display was read (fat content in µg/cm2 of the skin surface).
The results obtained were compared in µg/cm2 with parameters characteristic of healthy skin under normal conditions: temperature of 20°C, humidity of 40-60% (Table 6).
Table 6. Interpretation of sebometry results
Forehead, t-zone, scalp
Hair
Cheeks, eyelids, temple
Mouth corners, upper body, back, neck
Arms, hands, legs, elbows
Dry
less than 100
less than 40
less than 70
less than 55
0-6
Normal
100-200
40-100
70-100
55-130
0-6
Oily
more than 220
more than 100
more than 180
more than 130
---
2.4. Methods for injecting modified hyaluronic acid
To correct involutional changes in the skin, the patients were injected a preparation containing magnesium salt of HA (14 mg/ml) modified with vitamin C and amino acids (proline, lysine, glycine).
The drug was administered intradermally to a depth of 1.5-3 mm over the entire surface of the face, with a distance between injection points of 1 cm. The total volume of the drug administered once was 1.5 ml. The number of procedures was 3, the interval between procedures was 3 weeks.
The procedures were carried out as follows:
1. Application anesthesia with 5% Emla ointment 15-20 minutes before the procedure.
2. Skin disinfection with 0.5% solution of chlorhexidine bigluconate.
3. Conducting injections.
4. Skin disinfection with 0.5% solution of chlorhexidine bigluconate.
In a similar way, unmodified HA (gel with a concentration of 14 mg/ml) was administered to patients in the control group.
2.5. Methods of statistical processing of study results
Statistical processing of the material was carried out using the Statistica 6.0 for Windows software. Student's t-test was used to compare quantitative indicators before and after treatment. The criterion for statistical significance was p less than 0.05.
3.1. RESULTS OF A MORPHOLOGICAL STUDY OF SUBCUTANEOUS INTRODUCTION OF HYALURONIC ACID GELS UNMODIFIED AND MODIFIED WITH VITAMINS, AMINO ACIDS AND OLIGOPEPTIDES
To assess tissue response to the subcutaneous administration of HA unmodified and modified with vitamins, amino acids and microelements, a macroscopic, histological and histochemical study of tissues in the area of administration of HA hydrogels with various biologically active components was carried out – the 1st series of experiments.
5 options of hyaluronic acid gels were studied:
1. Native (unmodified) HA hydrogel (Gel No. 1)
2. HA hydrogel modified with vitamin C (Gel No. 2)
3. HA hydrogel modified with glutation (Gel No. 3)
4. HA hydrogel modified with folic acid (Gel No. 4)
5. HA hydrogel modified with vitamin C, proline, glycine, lysine (Gel No. 5)
HA content in the gels was 1.4 wt.% in all cases, the content of additives was 0.4 wt.%
The experimental studies were performed on 180 white laboratory rats. All studied drugs were administered subcutaneously. Tissues from the area of injection were studied in dynamics over the period from 1 to 14 days.
3.1.1 The results of macroscopic examination of subcutaneous gel injection sites
A day after the injection of the gels into the subcutaneous tissue, after dissecting the animals withdrawn from the experiment, it was visually established that gels No. 2 (modified with vitamin C) and No. 5 (modified with vitamin C, proline, glycine and lysine) were present at the injection site in the form of soft, transparent, compact, freely lying round formations with a volume of about 2/3 of the original. No signs of capsule. In the group of injection of gels No. 1 (unmodified HA without additives), No. 3 (modified with glutathione) and No. 4 (modified with folic acid), gel remains were found to be much smaller, no more than 1/3 of the original volume. They were transparent and looked like flat plates of irregular shape, about 3 mm thick.
On the 3rd day, gel No. 1 (native HA without additives) was not found at the injection site. The surrounding tissues had no visible changes. Thus, unmodified HA undergoes almost complete resorption during this period. In the groups of injection of gels No. 2 (modified with vitamin C), No. 3 (modified with glutathione) and No. 5 (modified with vitamin C, proline, glycine, and lysine), the macroscopic patterns were similar to each other. Hydrogels were found in the form of separate fragments of rounded shape, milky white color, soft consistency. They separated easily from the surrounding tissue. There is a slight increase in the vascular pattern.
After 7 days, the fatty tissue of the animals in the group with gel No. 2 (modified with vitamin C) included rounded whitish masses, dense to the touch, 3-4 mm in size, which were extracted together with the surrounding tissue. In group No. 3 (modified with glutathione), indurations of a brownish color were also found in the fiber. In groups with gel No. 4 (modified with folic acid) and No. 5 (modified with vitamin C, proline, glycine and lysine), an enhanced vascular pattern and several dense rounded formations 3-5 mm in size were detected at the injection sites. The surrounding tissue had areas of brownish color. These areas were extracted for histological examination.
On the 14th day, groups No. 2 and No. 5 had small foci of compacted fiber remaining in the gel injection zone. In the remaining groups, no gel was detected visually. The surrounding tissues had no visible changes.
3.1.2. Histological and histochemical study of tissue reaction and resorption of gel implants
Gel No. 1 (unmodified hyaluronic acid), 1 day after the injection, had a mostly loose structure due to watering (HA is very hydrophilic). The gel was represented by rare fibers, a network of very thin fibrils and fine granular material (Fig. 7).
Fig. 7. Gel No. 1 (unmodified HA), 1st day after administration. Hyaluronic acid gel consisting of fibers, a network of very thin fibrils and fine granular material. The gel shows neutrophils and macrophages, the latter show phagocytosed granules of HA. H-E staining. Magnification ×400
The gel is infiltrated by neutrophils and macrophages which are involved in the resorption of HA: neutrophils and macrophages secrete enzymes such as hyaluronidases that promote HA hydrolysis, and macrophages also phagocytize it. Staining with toluidine blue causes metachromasia, which is characteristic of acidic GAGs, which include HA (Fig. 8).
Gel fragments are located between the cells of the subcutaneous adipose tissue. Impregnation of adipose tissue with gel seems to be associated with the degree of molecular structuring and the high liquid content in the gel. Moderate edema, vascular plethora, and weak neutrophilic infiltration are noted in the surrounding tissues; this corresponds to weak tissue response.
Fig. 8. Gel No. 1 (unmodified HA), 1st day after administration. Metachromasia (purple staining) of fine fibrillar structures, indicating the presence of hyaluronic acid. Phagocytosis of the gel by macrophages. Stained with toluidine blue, ×1000 magnification.
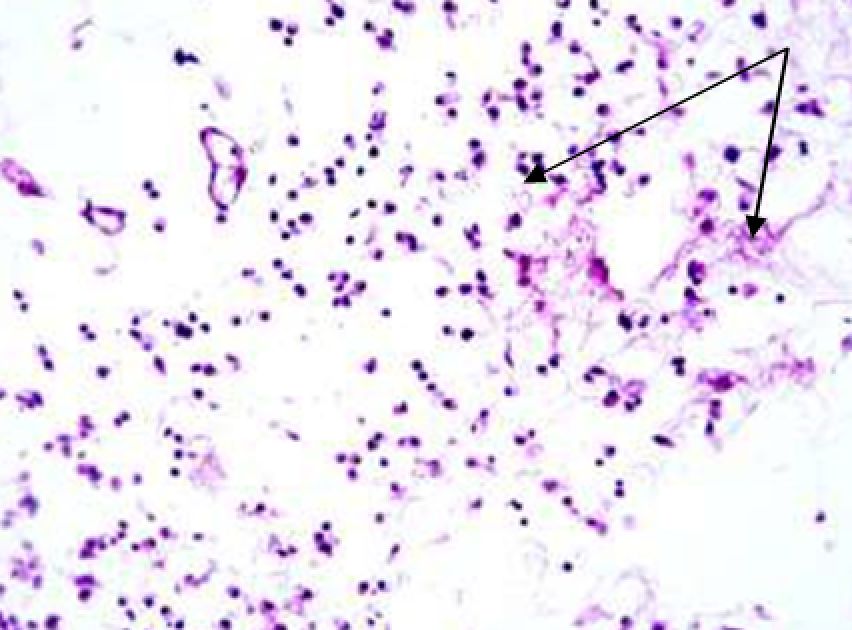
Fig. 9. Gel No. 1 (unmodified HA), 3rd day after administration. The gel reveals an uneven distribution of fine-fiber (felt-like) structures (shown by arrows). Ingrowing capillaries are visible at the top left. Neutrophil-macrophage infiltration remains H-E staining, ×200 magnification
3 days after implantation, the gel is no longer macroscopically detectable, but microscopy in the adipose tissue still shows some of its fragments. As in the 1st day, it has a fibrous structure and the fibers are intertwined with each other, taking on a felt-like structure (Fig. 9). The total gel content is significantly reduced compared to the 1st day. The number of neutrophils also decreased, but the content of lymphocytes and especially macrophages, which resorb the gel, increased. Thus, gel biodegradation occurs due to both cellular (mainly macrophage) resorption and cell-free enzymatic lysis. A few newly formed capillaries and fibroblasts are visible in the surrounding tissue, however no connective tissue capsule is formed around gel remains. Fibroblast response is weak.
On the 7th day, no gel was found in the biopsy specimen from the injection area. Only a small amount of perivascular lymphatic macrophage infiltration with single neutrophils and mast cells remains in the area.
Gel No. 2 (HA modified with vitamin C) After 1 day, the gel was macroscopically detected in the cellular tissue in the form of soft, transparent, compact, free-lying rounded formations with a volume of about 2/3 of the initial one. Its total amount in the tissue for this period was much more than that of gel No. 1 (unmodified HA). Histologically, unlike gel No. 1, it was a continuous conglomerate with a very loose fine-fibrillar structure, bordering on fatty tissue or superficial fascia. Two components are distinguished in the gel: loosened randomly weaving fibrous structures and a network of very fine fibers and granular material. The gel is infiltrated by resorbing neutrophils (to a lesser extent than gel No. 1) and macrophages (Fig. 10).
The gel partially impregnated the adipose tissue in which there was a weak inflammatory reaction with slight fibroblast proliferation. The gel was infiltrated mainly by neutrophils with some macrophages and lymphocytes.
On the 3rd day, macroscopically, the gel in the injection area was found as separate formations of a rounded shape, milky white color, and soft consistency.
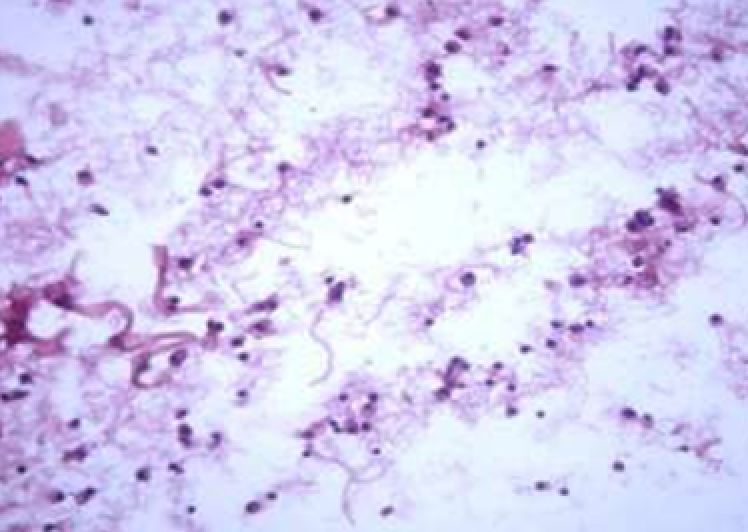
Fig. 10. Gel No. 2 (modified with vitamin C), 1st day after administration. Fibrous, fine-fibrillar and fine-granular gel structures, phagocytic macrophages and neutrophils. H-E staining. Magnification ×400
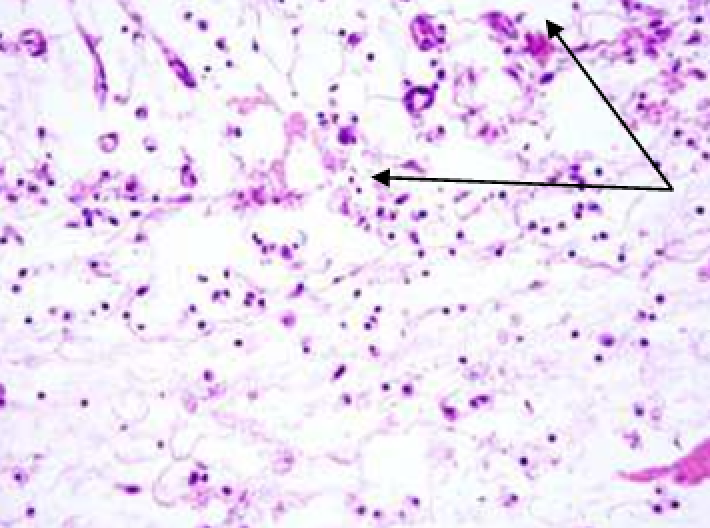
Fig. 11. Gel No. 2 (modified with vitamin C), 3rd day after administration. Below: gel with a large number of large macrophages, above: fatty tissue with plethoric vessels, cellular infiltration, and fibroblast proliferation (shown by arrows). H-E staining, ×400 magnification
Histologically, the gel was represented by large and small fragments in adipose tissue. Its content of fibrous structures decreased and the fine fibrillar network remained in the same volume.
The gel was infiltrated by cells, but now mainly by macrophages, which resorb and fragment it (Fig. 11). Gel lysis also occurs under the influence of hyaluronidase enzymes produced by macrophages and neutrophils.
A connective tissue capsule was not formed, but at the border of the gel and adipose tissue, in contrast to gel No. 1 (unmodified HA), there was increased proliferation of fibroblasts and new formation of capillaries, which was apparently associated with the effects of hyaluronic acid.
By the 7th day, the gel was subjected to lysis to an even greater extent. Separate fragments of the gel remained between the adipose and muscle tissue. In some places, the fibers of it were very loosened, while in other areas they were more compact.
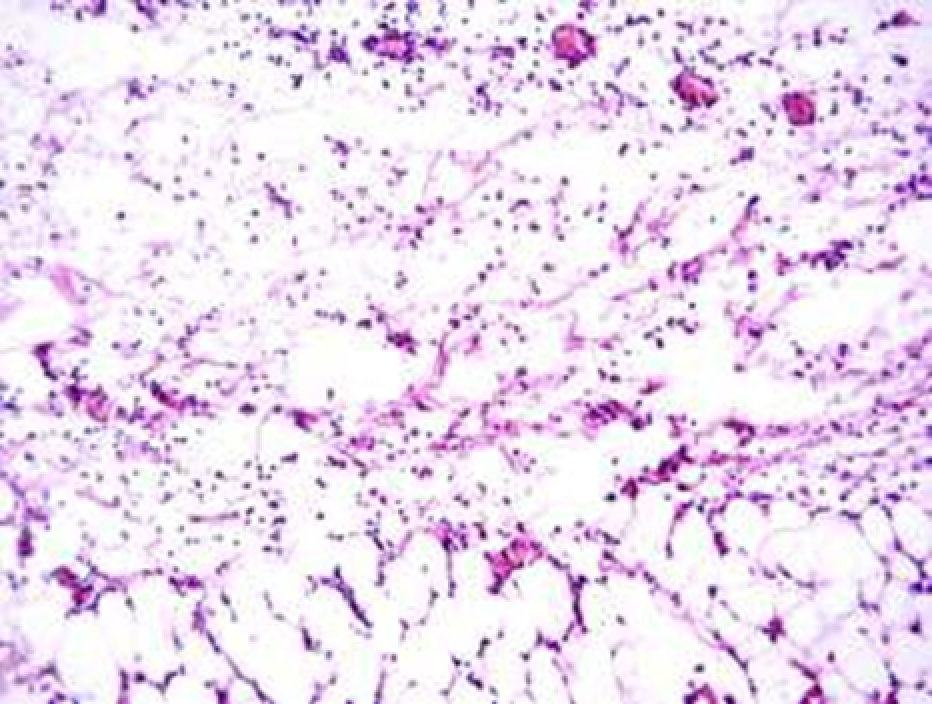
Fig. 12. Gel No. 2 (modified with vitamin C), 7th day after administration. Above: section of the gel with fibroblast and vascular invasion; below: adipose tissue with moderate infiltration by fibroblasts. H-E staining, ×200 magnification
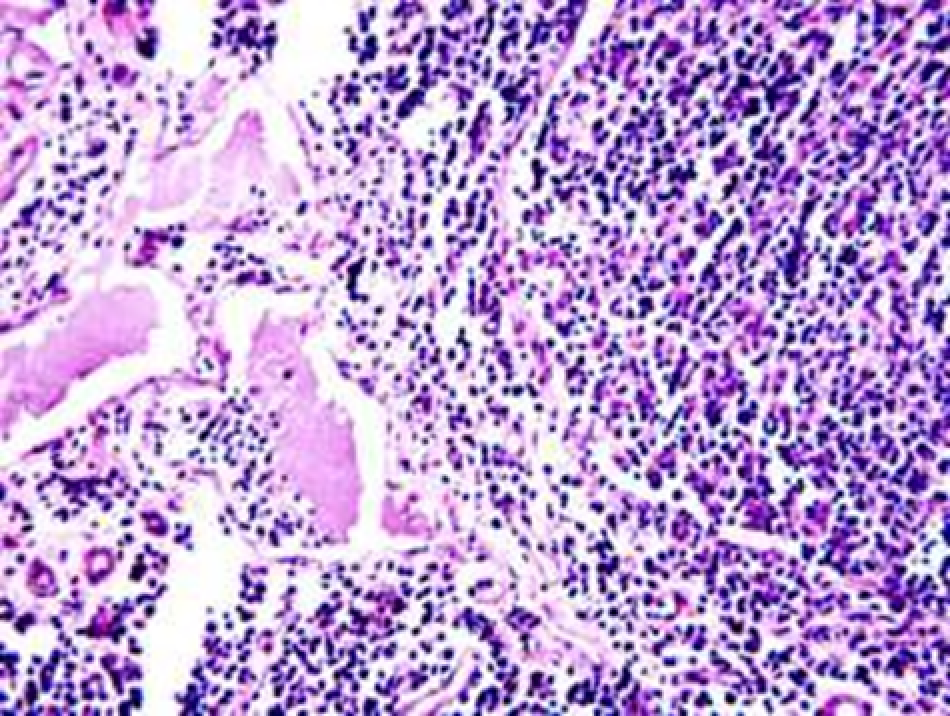
Fig. 13. Gel No. 2 (modified with vitamin C), 7th day after administration. Hyperplastic lymph node in the injection area. On the left, enlarged lymphatic cavities, on the right, a peripheral area of lymphoid follicles with a large number of macrophages. H-E staining, ×400 magnification
In some places, almost no distinct fibers are visible or they are single, but a network of fine fibrillar structures is still preserved. In other areas, especially near the adipose tissue, gel fibers are also preserved. The gel space is infiltrated with macrophages and partly with neutrophils to the same extent as on the 3rd day. The gel is ingrown with fibroblasts and full-blooded capillaries (Fig. 12), but the connective tissue capsule is still missing.
In the subcutaneous tissue in the injection area, there were scattered lymph nodes which are usually not found in rats in this anatomical zone, even when other gels are implanted. In the lymph nodes, there was hyperplasia of the lymphoid follicles and accumulations of macrophages phagocytizing the gel and transferred along the lymphatic pathways to these lymph nodes (Fig. 13).
After 14 days the gel is no longer detected macroscopically. During microscopy, there were small foci in the fatty tissue of some animals where the remains of gel imbibed the spaces between lipocytes. Small fragments of gel outside the tissue were also visible there, without any fibrous component in them, but fine fibrillar and fine granular one remained (Fig. 14).
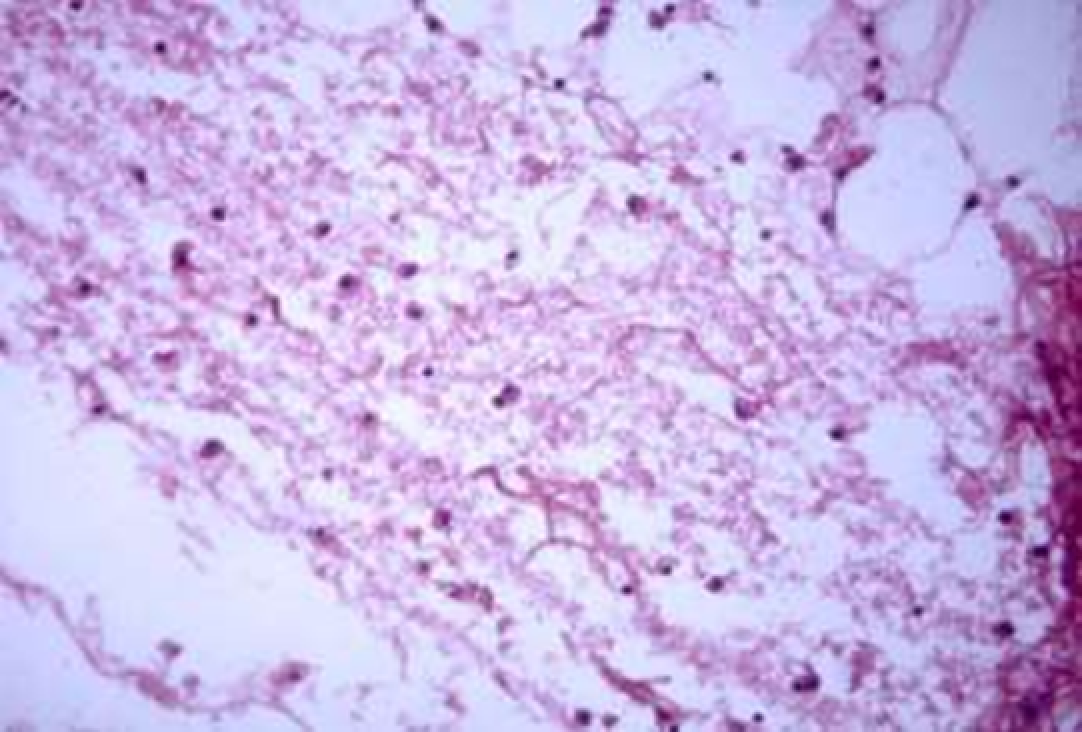
Fig. 14. Gel No. 2 (modified with vitamin C), 14th day after administration. Single fibers are visible in the gel, fine fibrils and fine granular matter prevail. Macrophages are phagocyting gel granules. No capsule. H-E staining. Magnification ×400.
Other animals still have small lympho-macrophage infiltrates and foci of fibroblast proliferation in the injection area.
Gel No. 3 (HA modified with glutation) After 1 day, the gel had the same structure as gel No. 2 (HA modified with vitamin C) – a combination of fibrous fine-fibrillar and fine-granular components (Fig. 15). Tissue response and microscopic picture were similar to those in the group with gel No. 1.
On the 3rd day, there was some difference. It was manifested by a lower degree of fibroblast and vessel ingrowth into the gel from the side of the surrounding tissues.
After 7 days, areas impregnated with gel having a fibrillar-vacuolized structure remained in the adipose tissue (Fig. 16). Ingrowth of fibroblasts and newly formed vessels was detected along the periphery of these areas. Neutrophils in the tissues were not numerous, but the content of macrophages was increased. Hyperplastic lymph nodes present in the gel injection zone had the same characteristics as with gel No. 2.
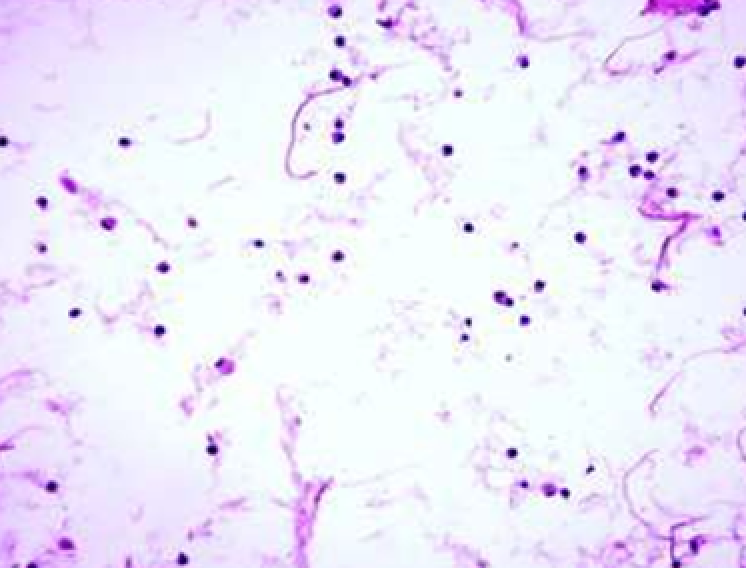
Fig. 15. Gel No. 3 (modified with glutation), 1st day after administration. Gel with loose fibrous structures of various thicknesses. H-E staining, ×400 magnification
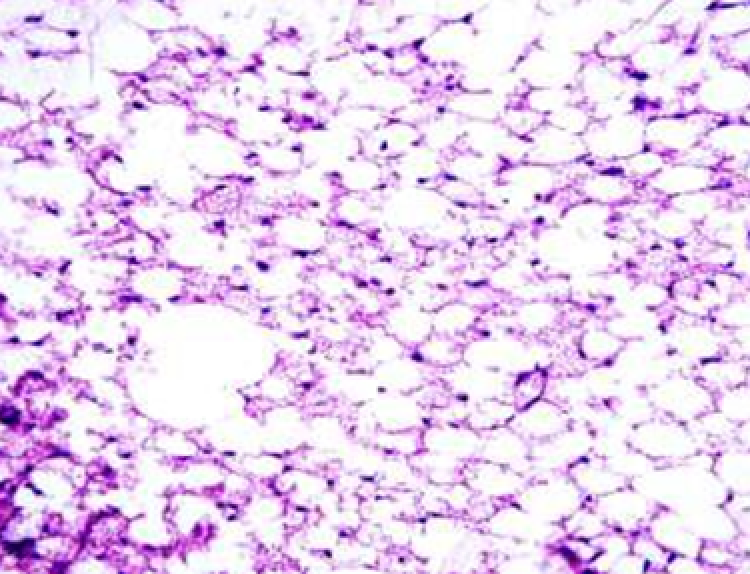
Fig. 16. Gel No. 3 (modified with glutation), 1st day after administration. Adipose tissue is impregnated with gel having a fine-vacuolar and fine-fibrillar structure. H-E staining, ×400 magnification
On the 14th day, the degree of macrophage reaction decreased and the proliferation of fibroblasts increased. The number of gel foci in adipose tissue and their size decreased significantly.
Gel No. 2 (HA modified with vitamin C) After 1 day, the microscopic picture is similar to that when using gel No. 2 (modified with vitamin C).
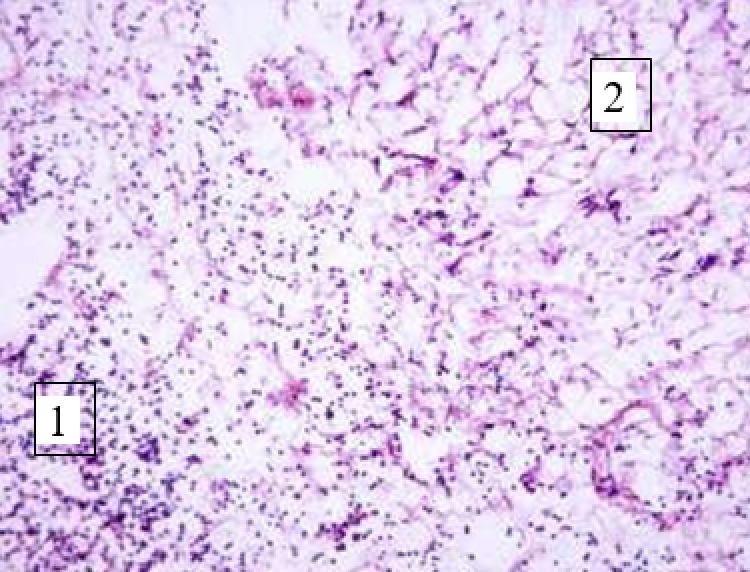
Fig. 17. Gel No. 4 (modified with folic acid), 1st day after administration: 1 - hyaluronic gel infiltrated with neutrophils, 2 - adipose tissue with moderate infiltration. No capsule present. H-E staining, ×200 magnification
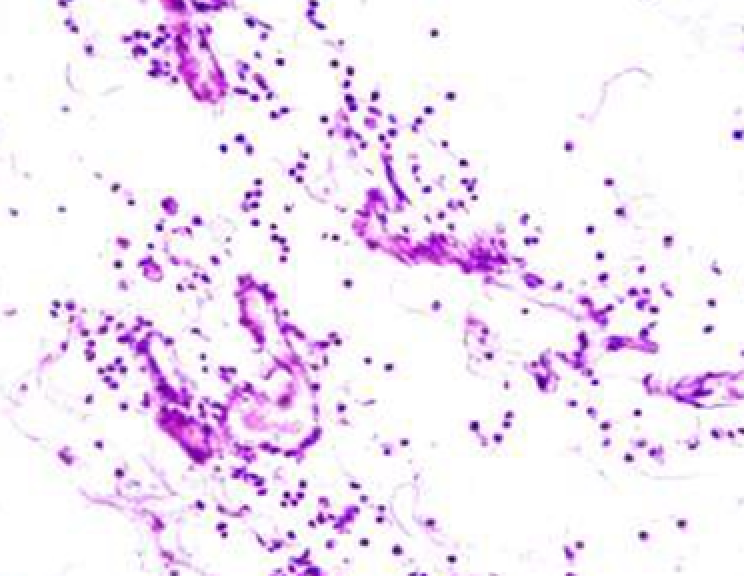
Fig. 18. Gel No. 4 (modified with folic acid), 3rd day after administration. Sharply edematous tissue penetrated by fine-fibrous gel elements. Germinating newly formed vessels and fibroblasts are visible. H-E staining, ×400 magnification
Part of the gel is represented by compact conglomerates, but there is no clear boundary between these formations and adipose tissue because the gel partially imbibes the adjacent tissue layers (Fig. 17). The content of neutrophils in the gel is slightly higher than in gel No. 2 (with vitamin C).
On the 3rd day, gel content in the conglomerate decreases, while in the fatty tissue it increases. The fibrous component in the gel decreases, and the macrophage reaction increases. At the periphery of the implant, in the tissue, vascular germination and fibroblast proliferation occur (Fig. 18).
By the 7th day, the growth of vessels and fibroblasts increases, but a capsule is not formed. The gel remains in fatty tissue in the form of small fragments only.
After 14 days, no gel is detected in the injection area, but the appearance of lymphoid follicles is noted (Fig. 19). Along the periphery of the follicles, numerous foci with macrophages are visible, having a vacuolized cytoplasm with a phagocytosed gel
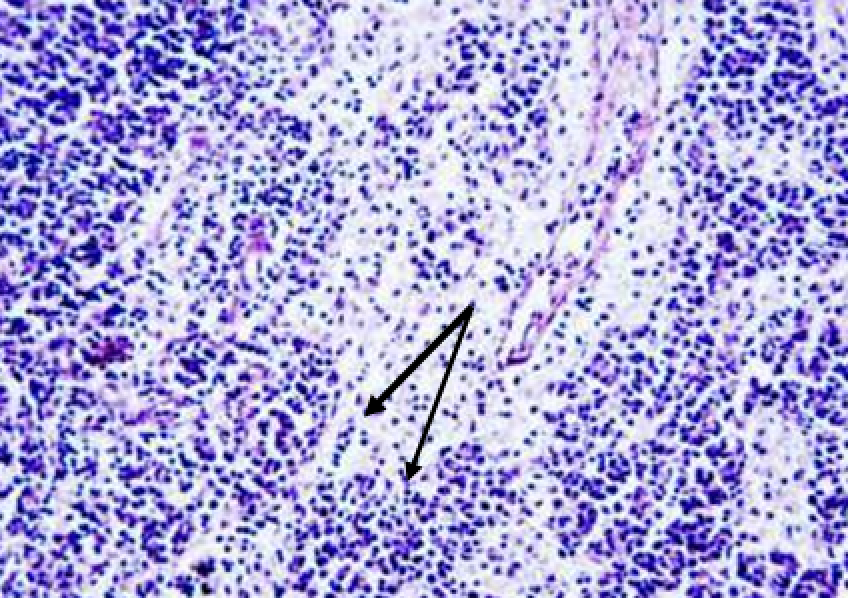
Fig. 19. Gel No. 4 (modified with folic acid), 14 days after administration. Lymph node near the injection site. On the periphery of the follicle, macrophages with phagocytosed gel particles in the cytoplasm are visible (arrows). H-E staining, ×400 magnification
Gel No. 5 (HA modified with vitamin C, proline, glycine and lysine). On the 1st day macroscopically the gel has a compact structure. Histologically, the gel, as in other groups, is loosened, but in some places it has denser areas where fine granular substance predominates (Fig. 20). The number and composition of cells in the gel is the same as in the group with gel No. 2 (modified with vitamin C). The surrounding tissues have vascular plethora and moderate infiltration by macrophages and neutrophils.
After 3 days, the gel decreased in volume but retained a compact structure. Loose areas alternate with dense ones, where the gel is condensed, especially along the periphery (Fig. 21). The gel is resorbed by macrophages. In the adipose fiber there is a moderate growth of fibroblasts and blood vessels.
By the 7th day, the growth of vessels and fibroblasts increases (Fig. 22), but the amount of gel decreases.
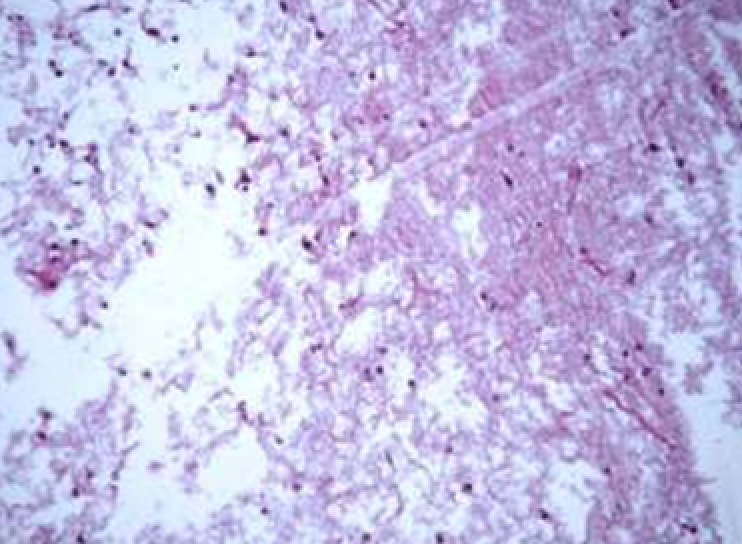
Fig. 20. Gel No. 5 (modified with vitamin C, proline, glycine and lysine), 1st day after administration. Central part of the implant. On the left - loosened, and on the right - condensed sections of the gel. H-E staining. Magnification ×200
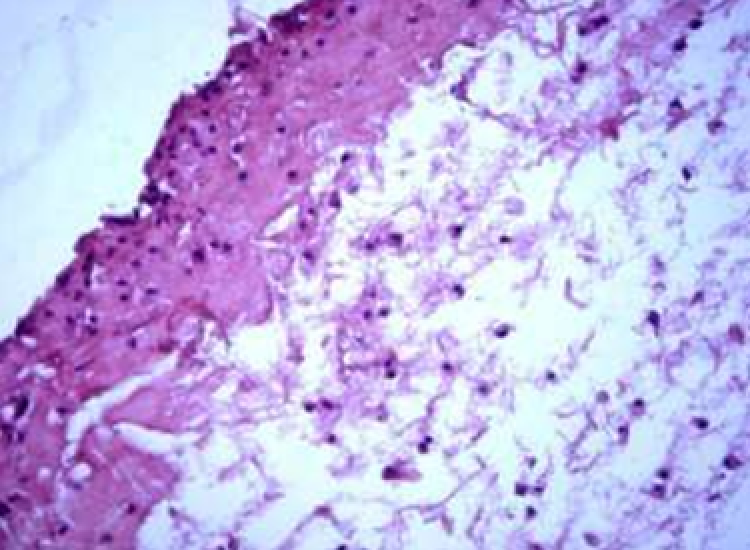
Fig. 21. Gel No. 5 (modified with vitamin C, proline, glycine and lysine), 3rd day after administration. Densifying of the gel in the marginal layer of the implant. H-E staining. Magnification ×400.
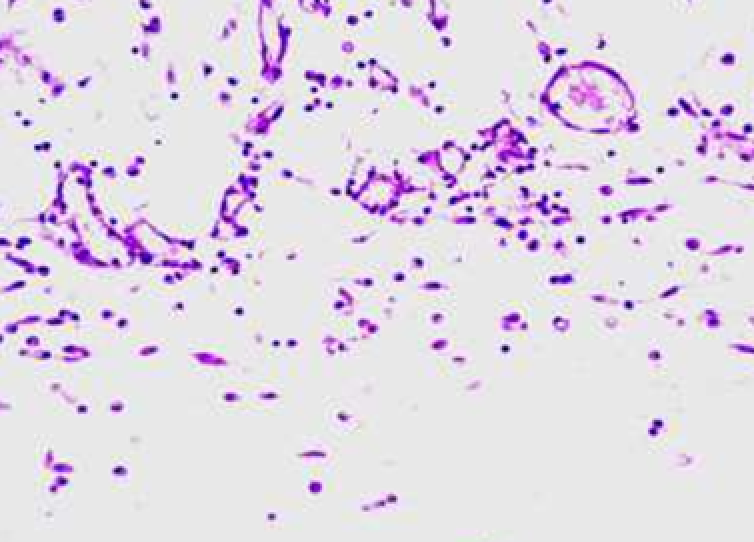
Fig. 22. Gel No. 5 (modified with vitamin C, proline, glycine and lysine), 7th day after administration. In adipose tissue at the border with the gel, there is an increased number of fibroblasts, macrophages and full-blooded microvessels.
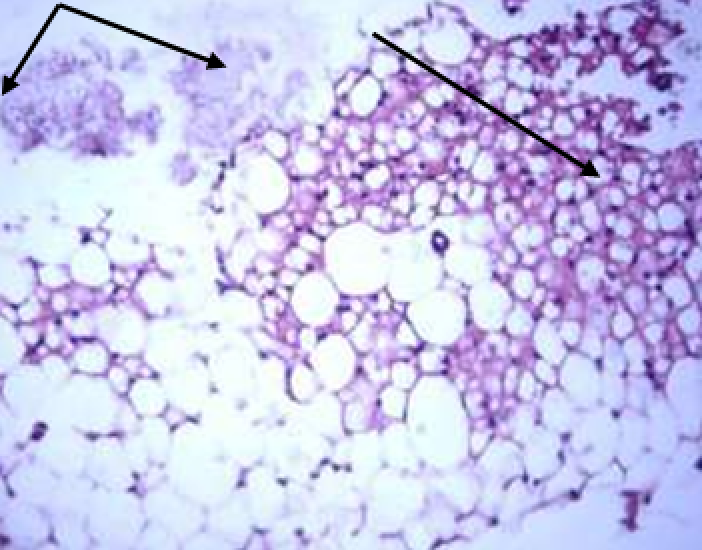
Fig. 23. Gel No. 5 (modified with vitamin C, proline, glycine and lysine), 14th day after administration. Top right – adipose tissue imbibed with gel (arrow), top left – fragment H-E staining, ×400 magnification of fibrillar-granular structure gel (angled arrow). H-E staining, ×400 magnification
On the 14th day, the gel was no longer detected in one animal, and in the other two, the remains of the gel imbibed fatty tissue (Fig. 23). One animal also had larger and denser gel fragments, and they lacked a fibrous component (Fig. 24).
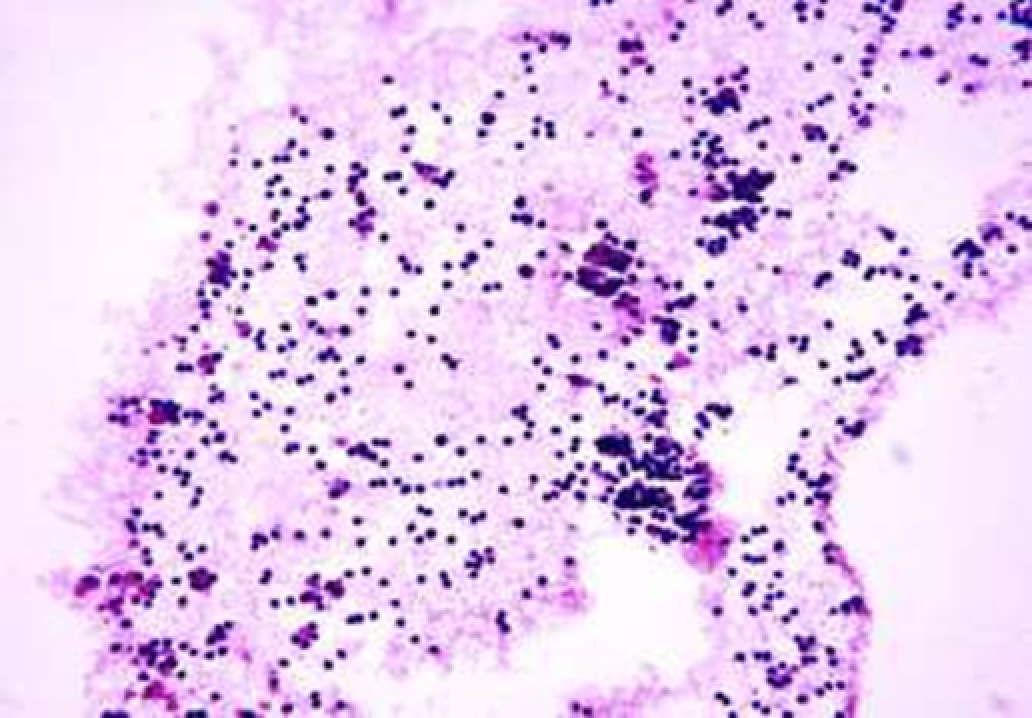
Fig.24. Gel No. 4 (modified with vitamin C, proline, glycine and lysine), 14 days after administration. The gel has fine-fibrillar, fine-grained and fine-vacuole structure. It shows lymphocytes, macrophages, and giant multinucleated cells. H-E staining, ×400 magnification.
In adipose tissue, foci of fibroblast proliferation and an increased content of blood vessels are found.
3.2. RESULTS OF A HISTOLOGICAL STUDY OF INTRACUTANEOUS INJECTION OF UNMODIFIED AND MODIFIED HYALURONIC ACID GELS
The next stage was to study the resorption and tissue reaction during intradermal administration of gels in which HA was subjected to solid-state modification with several biologically active substances - 2nd series of experiments.
3 options of hyaluronic acid gels were studied:
No. 1: native hyaluronic acid (control);
No. 2: HA hydrogel modified with vitamin C, glycine, proline, and lysine
No. 3:HA hydrogel modified with vitamin C and glutation.
3.2.1. Results of histological and histochemical studies
The intact skin of a pig, like that of other mammals, consists of the epidermis, papillary and reticular dermis. The epidermis is comparatively thin. It consists of 5 cell layers. In the basal layer, there are single cells with mitotic figures. The reticular dermis is thick, while the papillary layer is relatively thin. The reticular layer contains densely intertwined bundles of collagen fibers. The architectonics of collagen fibers corresponds to the skin of all mammals: it has a “basket weave” (Fig. 25). Part of the fibers in the manufacture of the section has longitudinal arrangement, and the other part is cut at an angle or perpendicular (Fig. 26).
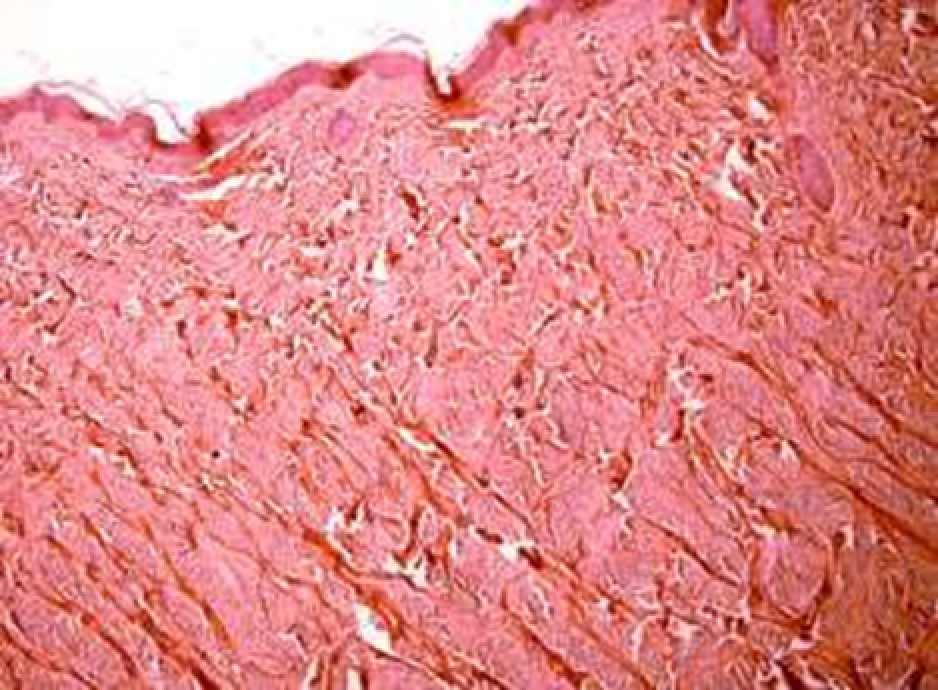
Fig. 25. Intact pig skin (control). Thin epidermis and thick reticular dermis. Staining according to Van Gieson, Х100 magnification.
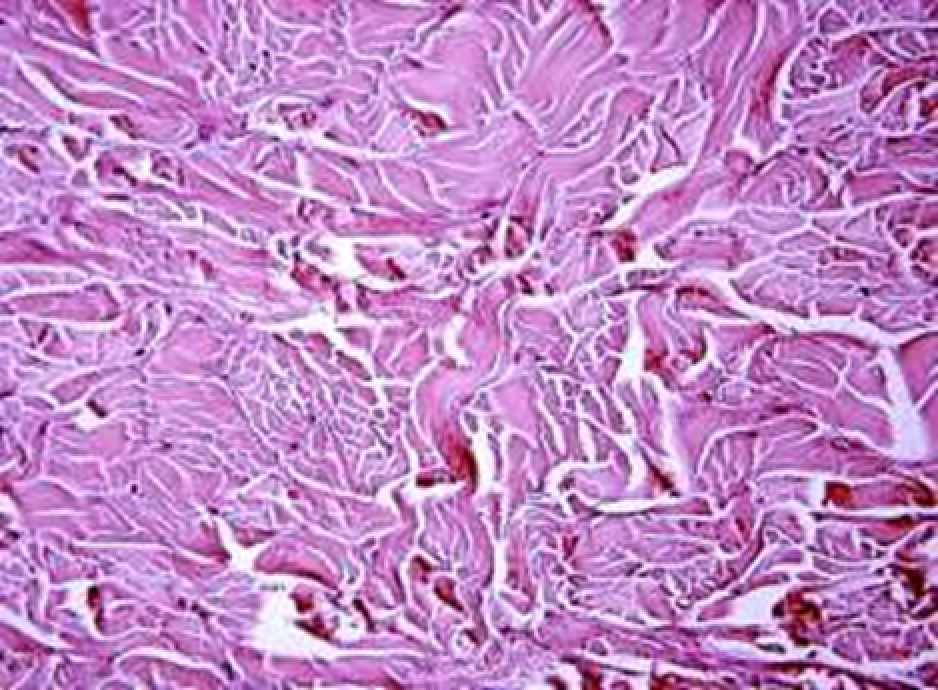
Fig. 26. Intact pig skin (control). Weaving of collagen fiber bundles characteristic of the dermis. Relatively rare cellular elements are fibroblasts. Hematoxyline and eozine staining, Х400 magnification
In the dermis, relatively rare capillaries, arterioles and venules are visible, and in the papillary layer there are more of these elements than in the reticular layer. In the dermis, hair follicles and sebaceous glands located near the follicles are found. Cellular elements in the dermis of the skin of the pig are few in number. They are represented mainly by fibroblasts, which have a spindled or stellate shape (see Fig. 26). In the papillary layer, in addition to fibroblasts, a few lymphocytes, macrophages (histiocytes) and mast cells are found. All these cellular elements are located mainly near the vessels.
On the 1st day after the injection, regardless of the composition of the injected gels, in all cases, the same type of changes were found in the epidermis and dermis, localized near the injection site (“injection point”). In the epidermis, these changes are represented by focal hyperplasia of the epithelium, its layer becoming several times thicker than in areas distant from the injection point (Fig. 27).
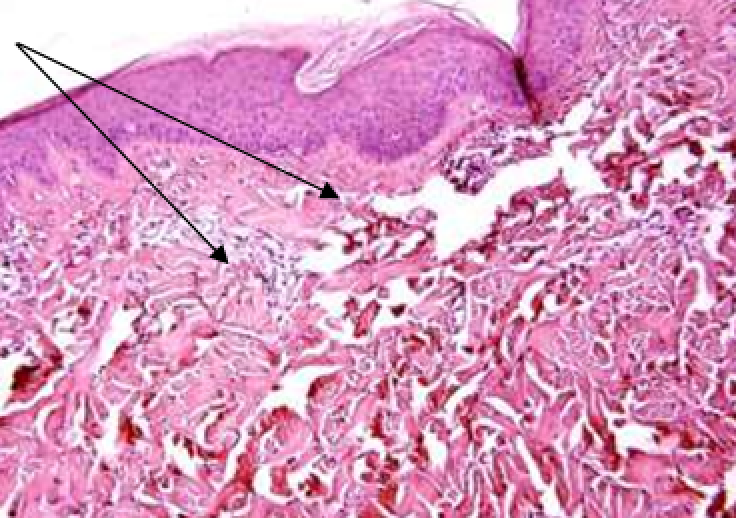
Fig. 27. Gel No. 1 (native HA), 1st day after administration. Hyperplasia of epidermis. Under the epidermis is the area where collagen fibers are loosened and HA vacuoles and newly formed capillaries are visible (arrows). Hematoxyline and eozine staining, Х 200 magnification
In the papillary and reticular layers of the dermis around the injection point, in all cases (all gel types), there was focal loosening of the collagen fibers. In the area of fiber loosening in groups with gels No. 2 (modified with vitamin C, glycine, proline and lysine) and No. 3 (modified with vitamin C and glutathione), small accumulations of proliferating fibroblasts are visible, as well as macrophage reaction. Macrophages surround the remnants of hyaluronic acid. The gels themselves almost do not stain, because already by this time they are mostly resorbed or spread between the fibers. At the site of injection of HA gels, small cells are formed with an increased content of macrophages and neutrophils around them, which are involved in HA resorption (Fig. 28). In other areas of the dermis, close to the injection site, foci of macrophage infiltration and the beginning of fibroblast proliferation are noted (Fig. 29).
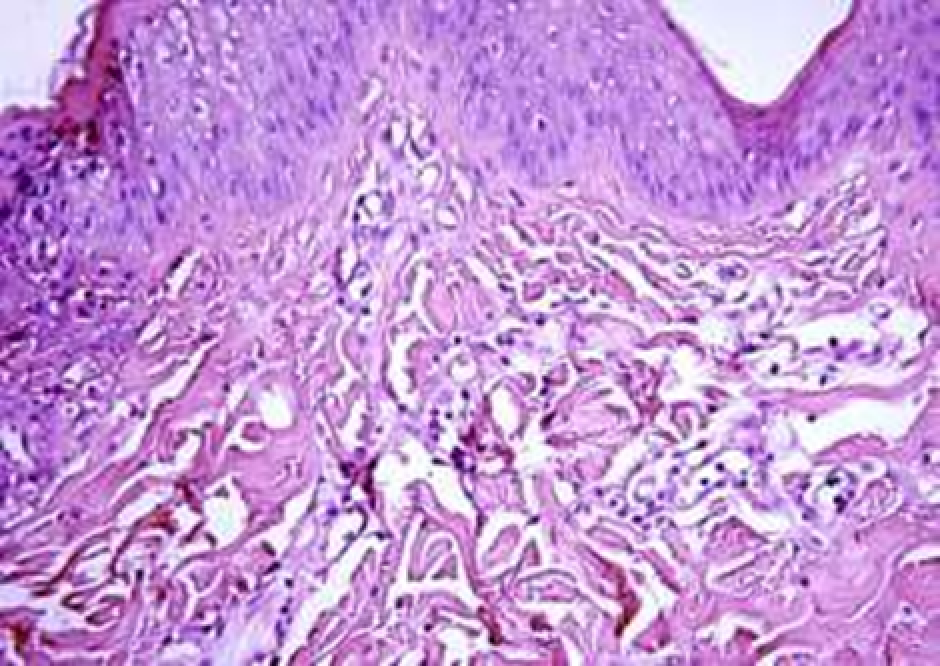
Fig. 28. Gel No. 2 (modified with vitamin C, proline, glycine and lysine), 1st day after administration. Loosening of collagen fibers, new formation of capillaries, infiltration by macrophages and neutrophils, proliferation of fibroblasts. Hematoxyline and eozine staining, Х 400 magnification
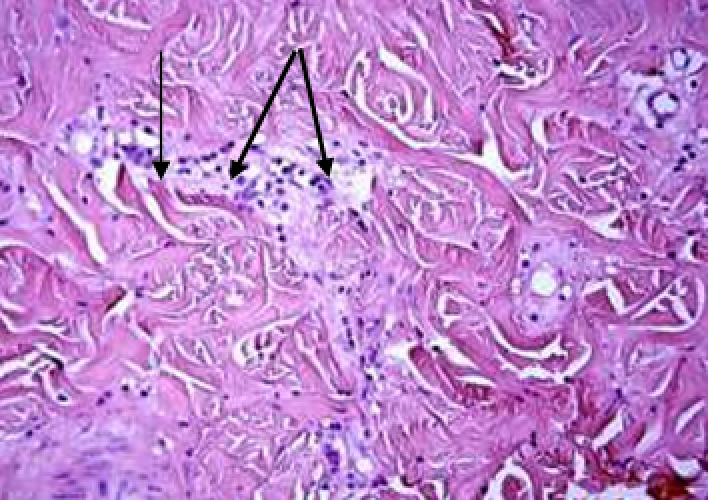
Fig. 29. Gel No. 3 (modified with vitamin C and glutatione), 1st day after administration. Remains of HA gel between collagen bundles (arrows). Macrophages, lymphocytes and proliferating fibroblasts are visible in this area. Hematoxyline and eozine staining, Х 400 magnification
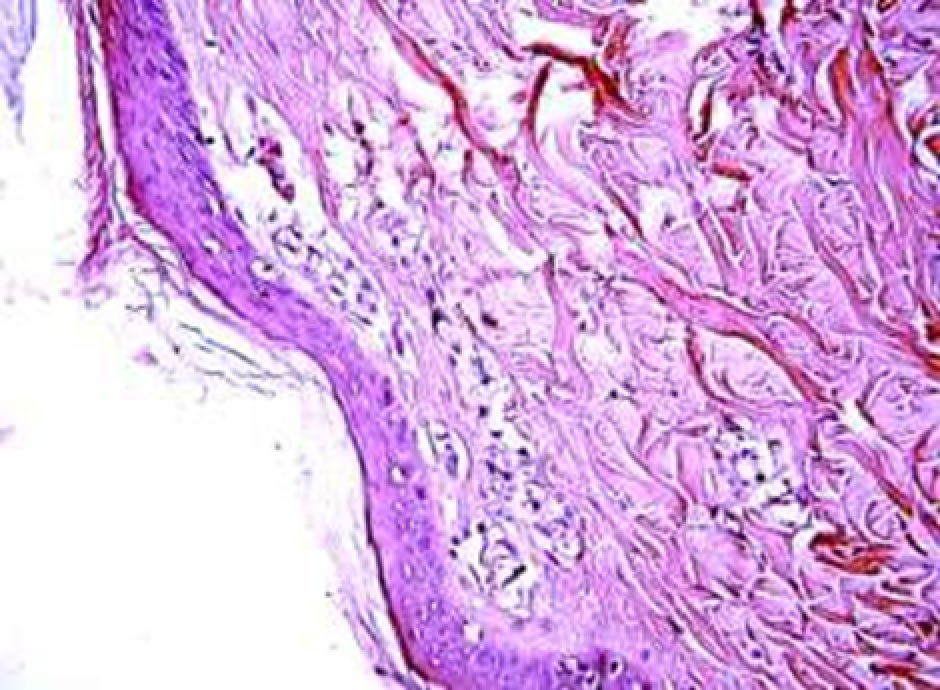
Fig. 30. Gel No. 3 (modified with vitamin C and glutatione), 3rd day after administration. Foci of gel residues with macrophage infiltration and fibroblast proliferation. Hematoxyline and eozine staining, Х 400 magnification
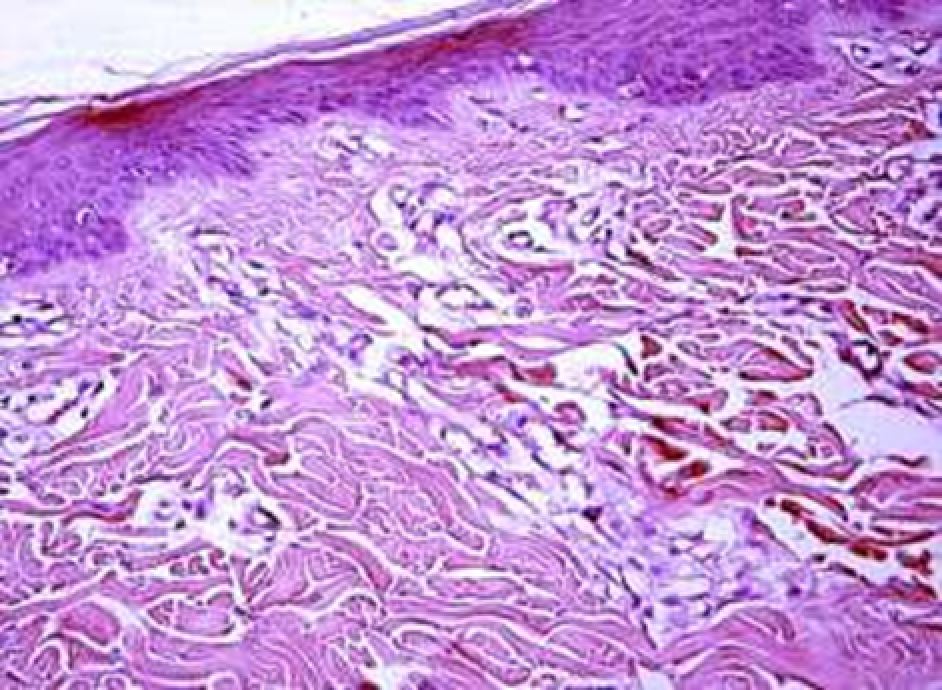
Fig. 31. Gel No. 2 (modified with vitamin C, glycine, proline, and lysine), 3rd day after administration. Foci of HA injection material replaced by capillaries, macrophage infiltrate and proliferating fibroblasts. Hematoxyline and eozine staining, Х 400 magnification
On the 3rd day after the administration of the gel, similar changes akin to those described in the previous period were also found in all groups. The difference lies in the decrease in the number of HA resorption foci. The proliferation of vessels and fibroblasts increases near the injection site, and in groups No. 2 (modified with vitamin C, glycine, proline and lysine) and No. 3 (modification with vitamin C and glutathione), there are more of them than in the control group (Fig. 30). Fibroblasts are characterized by an increased content of RNA in the cytoplasm. Some parts of the implant are replaced by fibroblasts, macrophages and vessels (Fig. 31).
In the case of using gel No. 2 (modified with vitamin C, glycine, proline and lysine), a rather large focus of non-resorbed gel of a mesh-cellular structure was found. The implant is being resorbed by macrophages (Fig. 32). In the case of administrating gel No. 3 (modified with vitamin C and glutathione), a relatively dense fragment of HA is also found in the thickness of the reticular layer of the dermis, with an immature microcapsule formed around it, consisting of fibroblasts and macrophages (Fig. 33).
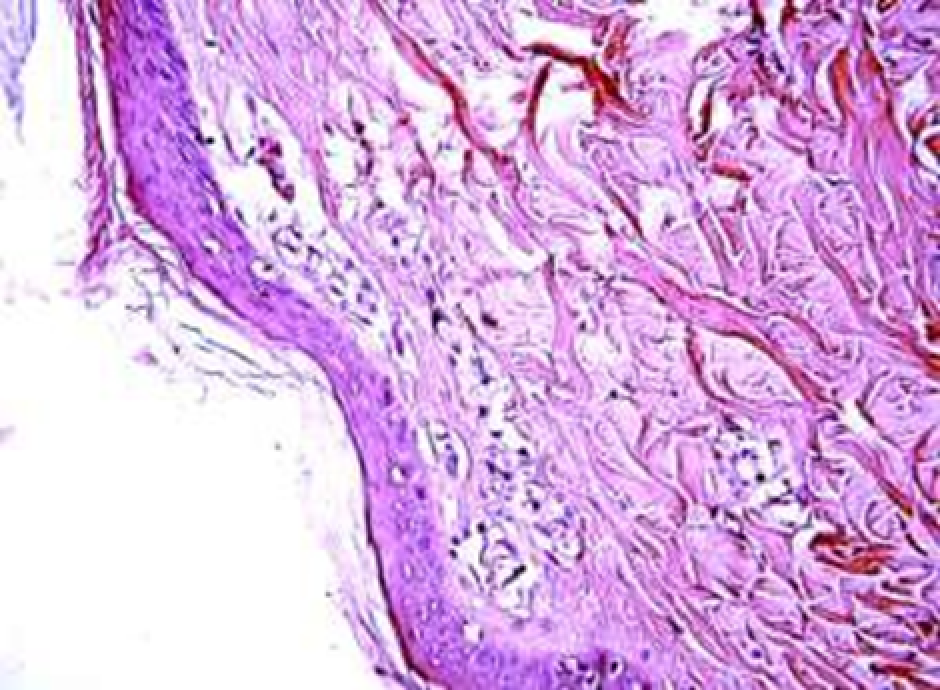
Fig. 32. Gel No. 2 (modified with vitamin C, glycine, proline, and lysine), 3rd day after administration A large focus of gel with a reticulate-cellular structure at the injection site. Macrophage resorption of the gel is visible. Hematoxyline and eozine staining, Х 400 magnification
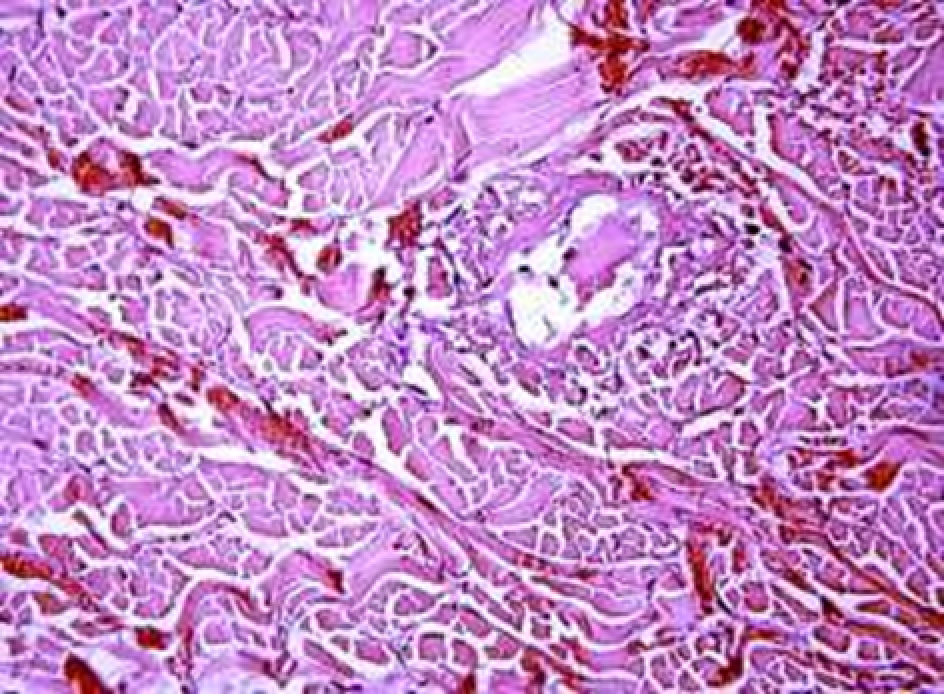
Fig. 33. Gel No. 3 (modified with vitamin C and glutathione), 3rd day after administration. Fragment of HA with macrophage resorption. The fragment is surrounded by a forming microcapsule. Hematoxyline and eozine staining, Х 400 magnification
On the 5th day after the injection of the gel, the animal’s skin in most biopsies (especially in the group with gel No. 1 – native HA) differs little from the intact one. The epidermis is thin, without any peculiarities in comparison with intact skin. In groups No. 2 (modified with vitamin C, glycine, proline and lysine) and No. 3 (modification with vitamin C and glutathione), there are few areas of fibroblast proliferation and vascular growth, which is a sign of dermis revascularization (Fig. 34). In one case (gel No. 2 – modification with vitamin C, glycine, proline and lysine), there is a section of the mesh-cellular structure, similar to that described for the 3rd day (Fig. 35).
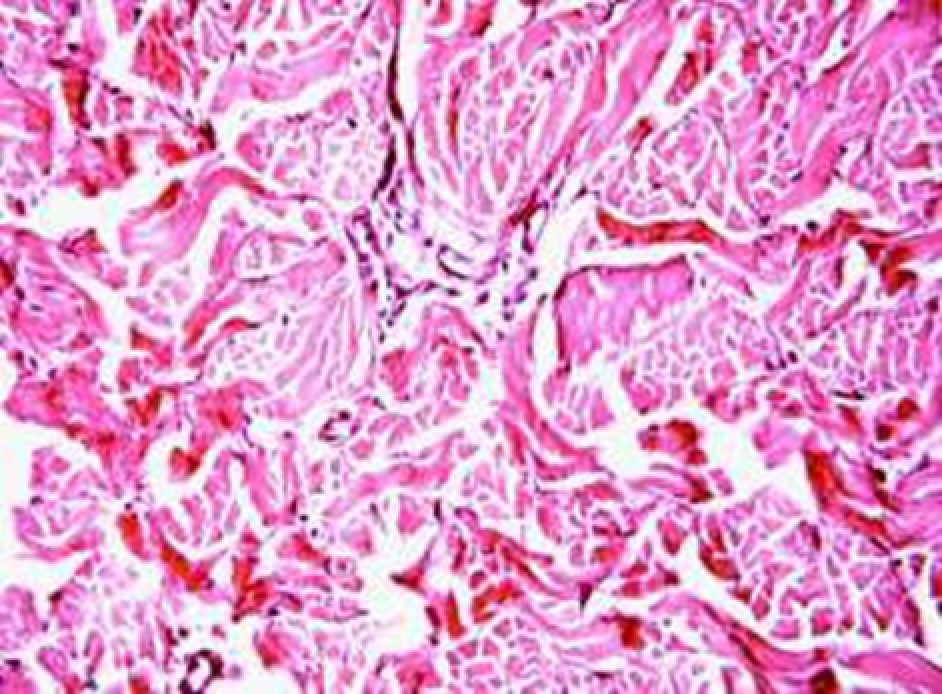
Fig. 34. Gel No. 2 (modified with vitamin C, proline, glycine and lysine), 5th day after administration. Increased number of microvessels and fibroblasts in the dermis, decreased macrophage response. Hematoxyline and eozine staining, Х 200 magnification
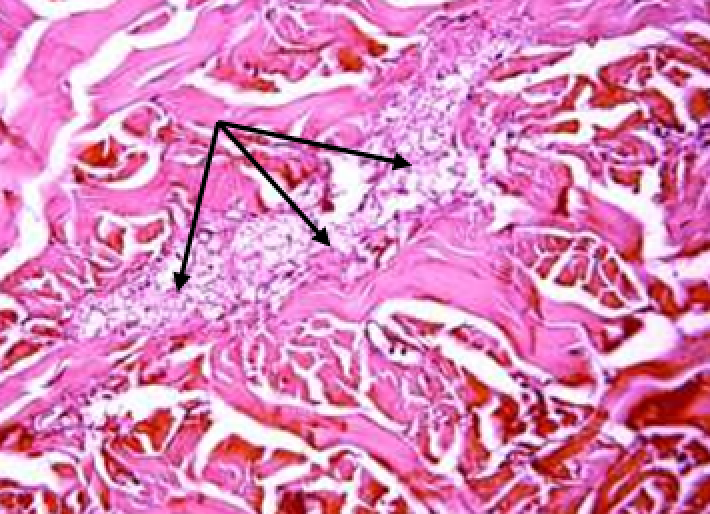
Fig. 35. Gel No. 2 (modified with vitamin C, proline, glycine and lysine), 5th day after administration. Mesh-cellular structure at the injection site of the gel (arrows) Hematoxyline and eozine staining, Х 400 magnification
3.2.2. Results of semi-thin sections study
Semi-thin sections (1-1.5 microns thick) were studied with the help of a light microscope in order to choose the site needed for electron microscopic examination on the 3rd day after the intradermal injection of various HA gels.
Just as in regular histological preparations, areas of change were found in semi-thin sections of animals that were intradermally injected with hyaluronic acid gels. They were located in the papillary dermis and on the border of the papillary and reticular layers. These areas (more often in groups No. 2, modification with vitamin C, glycine, proline and lysine) and No. 3, modification with vitamin C and glutathione) had empty thin-walled cells, in which, apparently, the gel was before, large macrophage cells involved in gel resorption, proliferating active fibroblasts, and mast cells with specific graininess in the cytoplasm (Fig. 36).
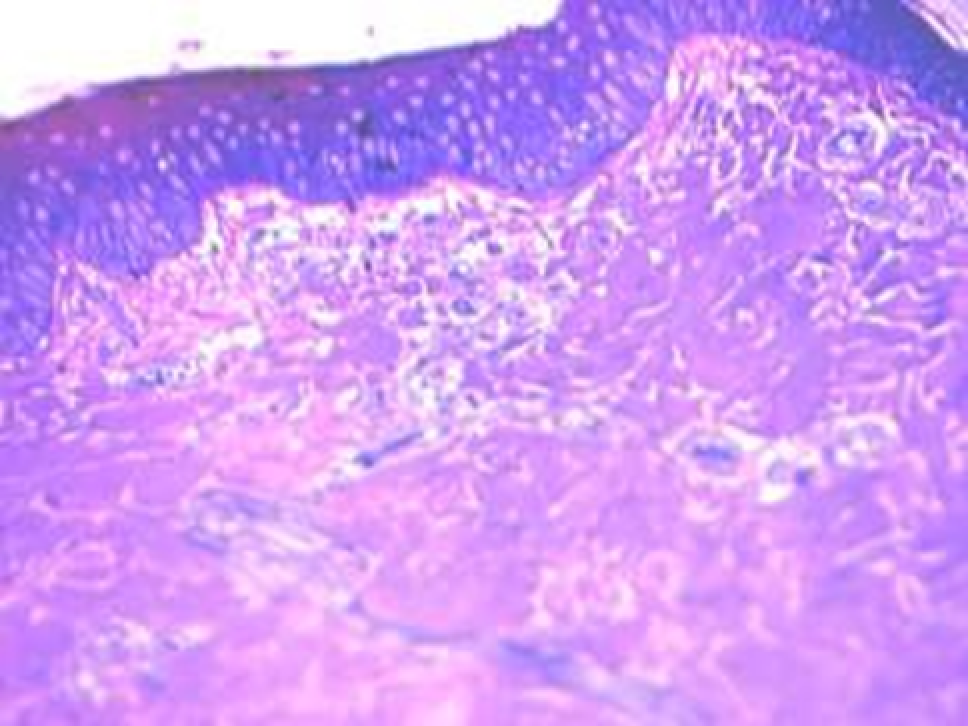
Fig. 36. Gel No. 2 (modified with vitamin C, glycine, proline, and lysine), 3rd day after administration. At the injection site of the gel, there are empty cells, various cellular elements and vessels. Stained with toluidine blue, Х400 magnification
In the same areas, the presence of newly formed capillary vessels significantly increases.
In groups with gels No. 2 and No. 3, foci of preserved gel are also detected (Fig. 37).
In one case, of gel No. 2 (modified with vitamin C, glycine, proline and lysine), a focus of a relatively dense gel with reticulate structure was found (Fig. 38).
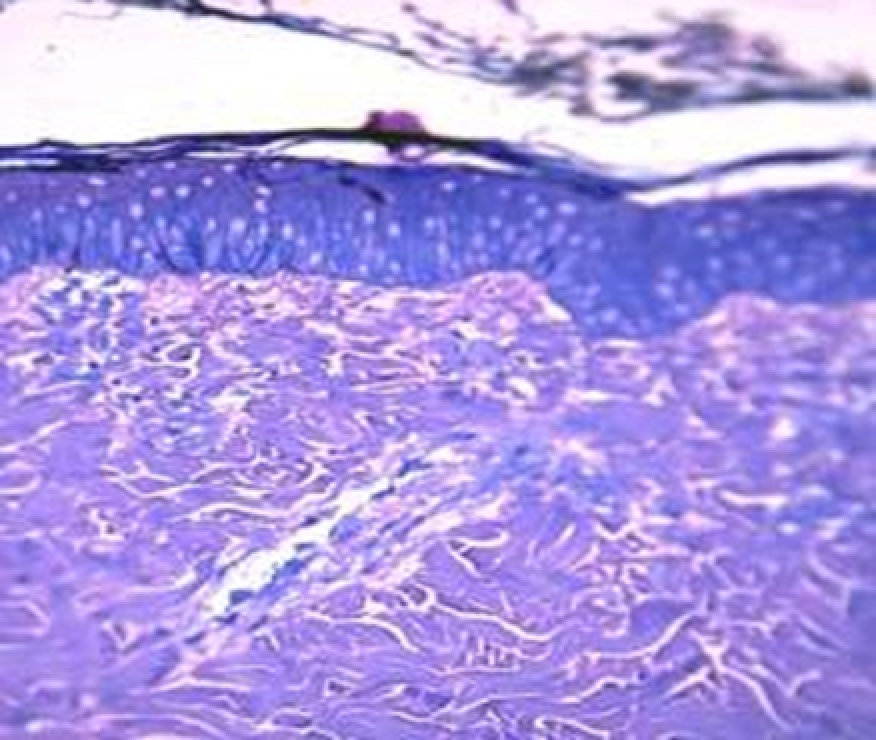
Fig. 37. Gel No. 3 (modified with vitamin C and glutathione), 3rd day after administration Foci of gel germinating with macrophages, fibroblasts and vessels. Stained with toluidine blue, Х400 magnification.
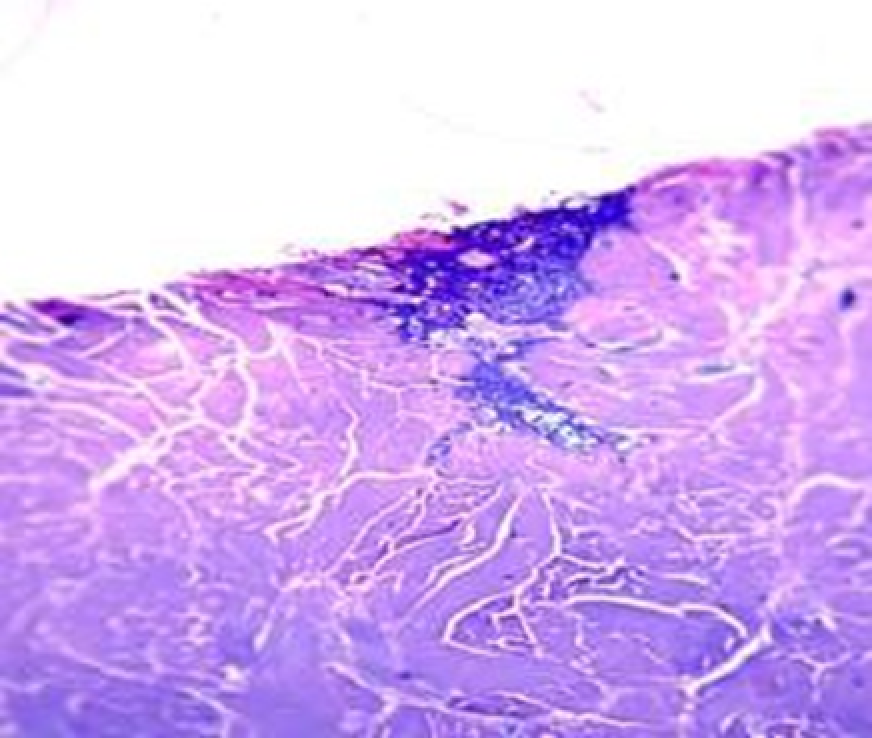
Fig. 38. Gel No. 2 (modified with vitamin C, glycine, proline, and lysine), 3rd day after administration. The center of the gel mesh-cellular structure. Stained with toluidine blue, Х400 magnification.
3.2.3. Transmission electron microscopy
Electron microscopic examination of pig skin biopsies on the 3rd day after intradermal injection of hyaluronic acid gels confirmed and deepened the data obtained by light microscopy. Thus, the increased content of RNA in the cytoplasm of fibroblasts actively proliferating in the foci of gel resorption, especially in groups No. 2 (modified with vitamin C, glycine, proline and lysine) and No. 3 (modified with vitamin C "and glutathione), found during histological examination, is confirmed by ultrastructural signs of increased protein synthesis: a well-developed granular endoplasmic reticulum and Golgi complex (Fig. 39, 40 and 41).
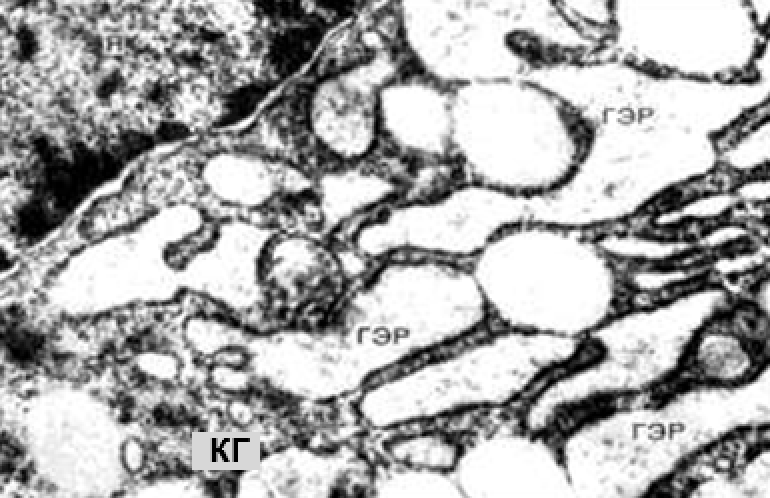
Fig. 39. An electron diffraction pattern of pig skin on the 3rd day after the introduction of gel No. 2 (modified with vitamin C, glycine, proline and lysine). Active mature fibroblast, hyperplasia of the endoplasmic reticulum (ГЭР) and Golgi complex (КГ) x25,000 magnification
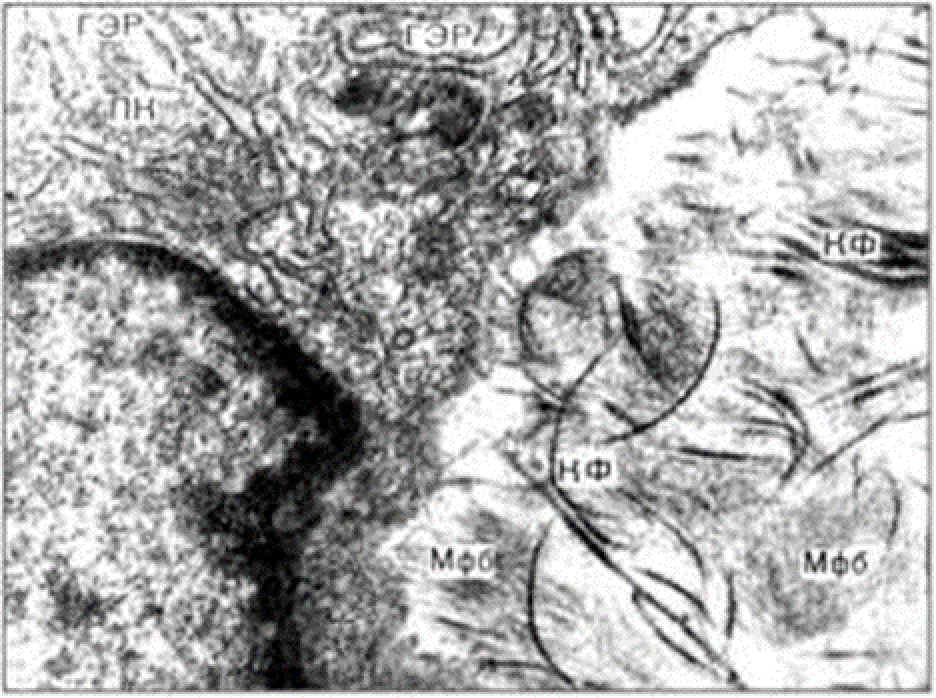
Fig. 40. Electron diffraction pattern of pig skin on the 3rd day after the introduction of gel No. 3 (modified with vitamin C and glutathione). Fibroblast with pronounced endoplasmic reticulum hyperplasia. Near the fibroblast, fibrillogenesis of newly formed collagen fibrils is noted. x15,000 magnification
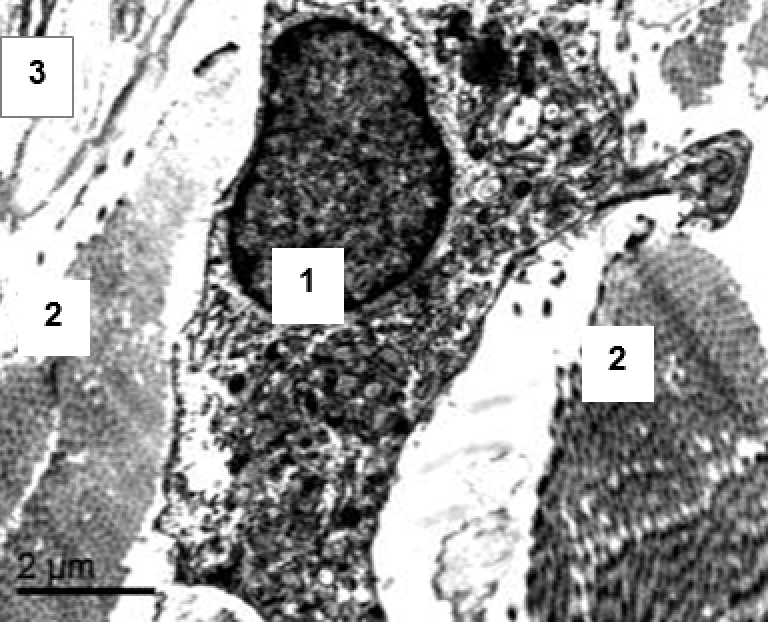
Fig. 41. An electron diffraction pattern of pig skin on the 3rd day after the introduction of gel No. 2 (modified with vitamin C, glycine, proline and lysine). In the center: active fibroblast with numerous organelles (1). Dense bundles of collagen fibrils in oblique and cross sections (2). Elastic fiber (3). x12,000 magnification
A significant macrophage reaction revealed in the experiment, including phagocytic activity by macrophages, manifests itself in an increased content of phagosomes and lysosomes in their cytoplasm (Fig. 42).
Electron microscopy revealed an increased number of mast cells. Their cytoplasm contains a large number of specific granules of varying degrees of maturity (Fig. 43).
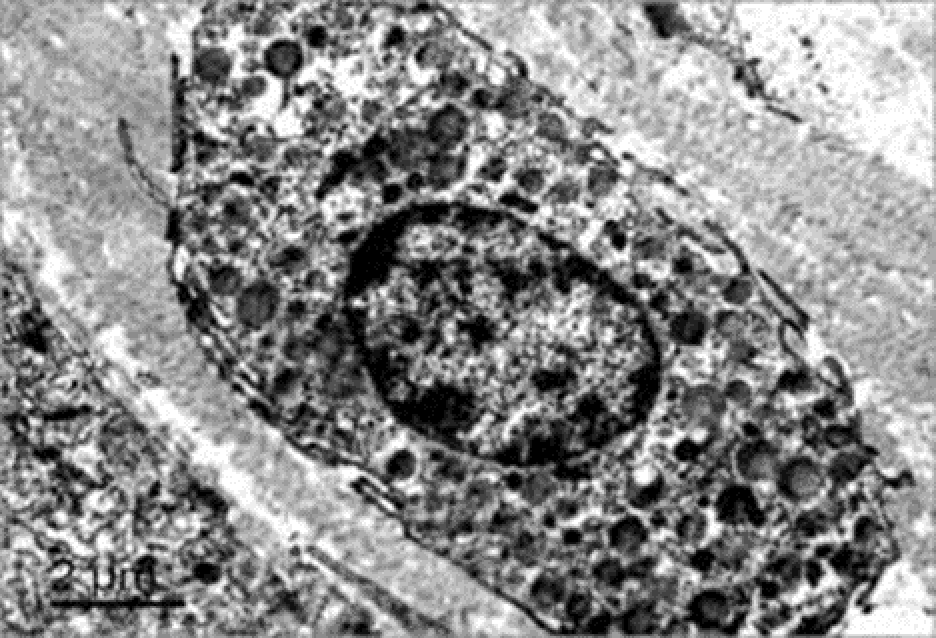
Fig. 42. An electron diffraction pattern of pig skin on the 3rd day after the introduction of gel No. 2 (modified with vitamin C, glycine, proline and lysine). Macrophage with increased content of phagosomes and lysosomes. x10,000 magnification
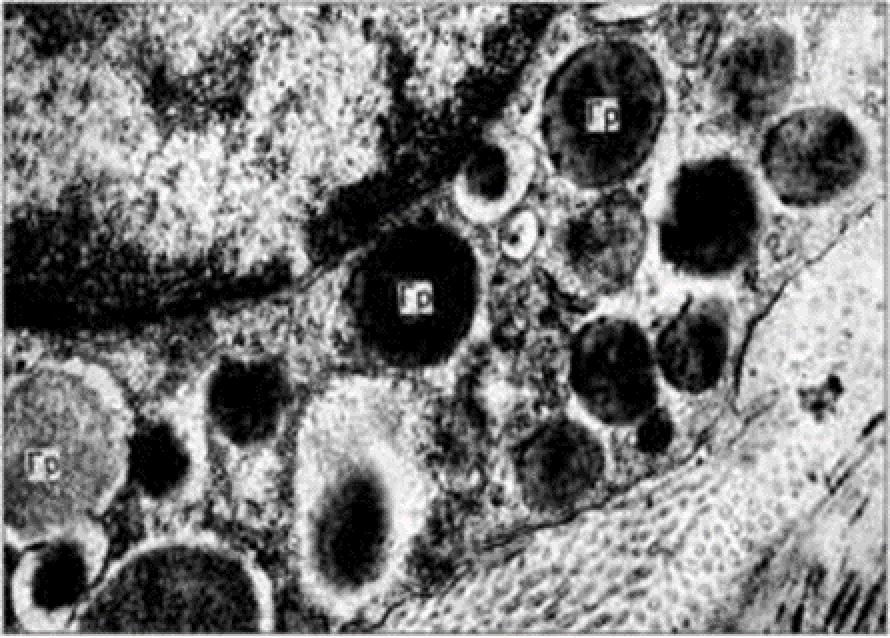
Fig. 43. Electron diffraction pattern of pig skin on the 3rd day after the introduction of gel No. 3 (modified with vitamin C and glutathione). Mast cell with high content of granules in the cytoplasm. The granules have different degrees of maturity. x10,000 magnification
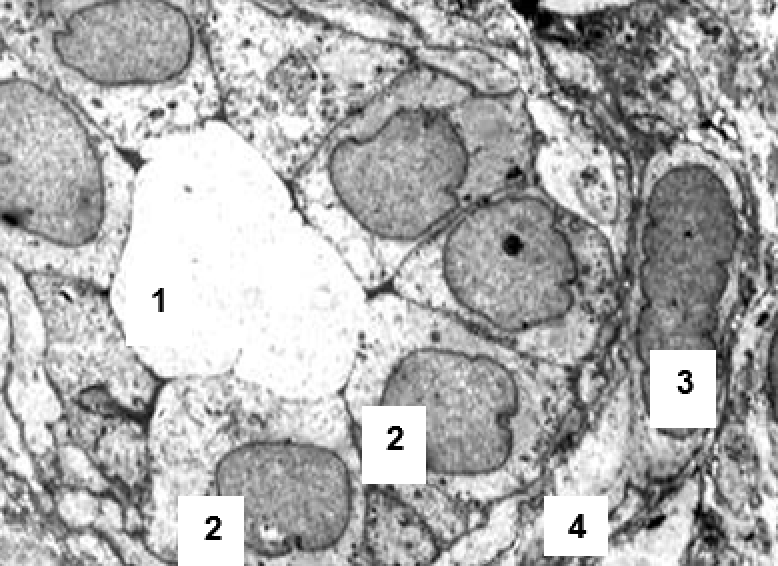
Fig. 44. Electron diffraction pattern of pig skin on the 3rd day after the introduction of gel No. 3 (modified with vitamin C and glutathione). Newly formed capillary without pathological changes: 1 - lumen 2 - endothelial cells 3 – pericyte 4 – basement membrane Magnification X8000
The capillaries found by EM have the regular ultrastructure of newly formed capillaries: endothelium, pericytes, basement membrane (Fig. 44).
This histological, histochemical and electron microscopic study of the dynamics of resorption of native and modified HA gels, as well as the development of tissue reactions during their subcutaneous and intradermal administration, performed on experimental animals was intended to provide an experimental substantiation of the advantages of such intradermal injections in patients with involutional skin changes.
Since an experimental model of intradermal administration of gel compositions in rats, in particular HA gels, is difficult to implement due to the anatomical features of the skin structure, a model of subcutaneous gel administration was used for the first stage of research. In the first series of experiments performed on 180 white laboratory rats, 5 variants of HA gels were studied: native (unmodified) and modified with various biologically active substances (BAS). The main objectives of this series of experiments were to evaluate the effect of the studied gel compositions on tissues in their administration area, identify features and patterns of tissue reaction development, and select the most effective materials for further experimental studies of their intradermal administration, modeled on minipigs.
The choice of biologically active substances to modify HA was based on literature data and the results of our own preliminary studies. The selection criterion for biologically active substances was determined by their high antioxidant effect or stimulating effect on skin regeneration. In addition, all the ingredients used to modify HA for the purposes of this study are classic geroprotectors. Literature has information about vitamin C’s ability to increase the formation of GAGs, in particular HA and chondroitin sulfate, and to stimulate the proliferation of fibroblasts. The physiological effect of vitamin C is associated not only with the activation of collagen production, but also with the decrease in the production of metalloproteinases, enzymes that destroy dermal collagen (141). Many studies have confirmed the ability of ascorbic acid to improve the condition of the skin and maintain its health, particularly effectively combating the primary signs of aging (54, 100).
The aminoacids glycine, proline, and lysine form part of the basic proteins of the derm’s intercellular matrix. Their presence is necessary to trigger the synthesis of the body’s own collagen and elastin, which is extremely important to achieve a stable, long-term effect (41).
The tripeptide glutathione is a powerful antioxidant that acts at various stages of the free radical chain process of biomolecule oxidation. In the body, glutathione in combination with cysteine restores the oxidized form of vitamin C to its original active form (41).
Morphological studies of biopsy specimens of animal skin and soft tissues in the first series of experiments showed that the lysis of native (unmodified) HA gel (gel No. 1) occurred much faster than that of HA gel modified with BAS. This seems to be related to the lower degree of structuring in unmodified HA. All types of gels, but especially gel
No. 1, imbibed the surrounding fatty tissue starting from the first day. By days 3-7, this process intensified, and the amount of free-lying gel decreased progressively. Gel lysis occurred due to cell resorption (phagocytosis by macrophages), as well as to the action of hyaluronidases produced by macrophages and neutrophils. The native (non-modified) HA gel was not found macroscopically (visually) in the injection zone as early as on the 3rd day, and histologically it was represented by small fragments in the fiber. By the 7th day, gel No. 1 was completely resorbed.
At the border between the modified gels and adipose tissue, the proliferation of fibroblasts and new formation of capillaries gradually increased. This process was especially pronounced in the case of gels No. 2 (HA modified with vitamin C) and No. 5 (HA modified with vitamin C, proline, glycine and lysine). Fibroblasts and capillaries grew into the gel, however, the connective tissue capsule around the implant was not formed until the end of the experiment (14 days). This is the main feature of this type of gels, indicative of their high bioinertness.
The presence of hyperplastic lymph nodes in the area of injection of some gels, like No. 2 (modified with vitamin "C") and No. 4 (modified with folic acid), is another distinguishing feature. Apparently, this may be due to these gels’ weak structure, allowing them to quickly enter the lymphatic collectors, penetrate through them into lymph nodes and induce their hyperplasia.
Gel No. 4 (modified with folic acid) underwent complete lysis by day 14, but cell proliferation in the surrounding tissues remained. However, hyperplastic lymph nodes appeared near the injection points. In them, we detected macrophages phagocytizing the gel and also elements of the gel itself.
HA gels with vitamin C and glutathione remained in the injection zone in the form of fragments in adipose tissue. But the longest-living one was gel No. 5, containing vitamin C and a complex of amino acids.
Thus, the experiments performed showed the efficiency of modified HA hydrogels, especially of those containing vitamin and amino acid complex (No. 5) compared to unmodified HA.
In case of intradermal administration, just as in that of subcutaneous administration, gels, due to their fluidity, “spread” within a day in small fragments between bundles of collagen fibers, forming mesh-cellular structures. The infiltration of gels with neutrophils is reduced compared with subcutaneous administration. Revealed mesh-cellular structures on the 1st day were still relatively numerous, but in subsequent periods, on the 3rd and especially the 5th day, their number noticeably decreased, because. gels underwent macrophage resorption and cell-free lysis under the influence of tissue hyaluronidases. Residues of HA gels modified with vitamin C and a complex of amino acids or vitamin C with glutathione, are found after 5 days in the form of small vacuoles and larger reduced reticulate-cellular structures. Native HA gel is no longer detected during this period.
Already on the first day after administration, all HA gels undergo macrophage attack, fibroblasts proliferate in these areas and newly formed capillaries penetrate. After 3-5 days, fibroblast proliferation and capillary growth increase, which can be understood as revascularization of the skin dermis.
The study of the ultrastructure (transmission electron microscopy) confirmed the findings obtained by light microscopy of histological sections. Numerous macrophages with a large number of lysosomes and phagosomes were found, which indicated enhanced phagocytosis of the gel. Fibroblasts have ultrastructural features, indicative of active biosynthesis of collagen and elastin. Collagen and elastin fibrillogenesis is clearly detected in the intercellular matrix of the skin. Skin neoangiogenesis is characterized by numerous newly formed capillaries of normal ultrastructure.
Comparative analysis shows that fibroblast proliferation, collagen, elastin synthesis and neoangiogenesis are more pronounced with the introduction of the HA gel modified with vitamin C with glycine, proline and lysine, than with the introduction of native HA.
Based on electron microscopy studies, it should be assumed that intradermal injection of HA gels modified with vitamin C, amino acids and oligopeptides in patients with involuting skin changes improves skin metabolism and enhances the structure of the collagen-elastic skeleton without local fibrosis.
3.3. STUDY OF CLINICAL AND MORPHO-FUNCTIONAL SKIN PARAMETERS IN PATIENTS OF DIFFERENT AGES BEFORE AND AFTER INJECTIONS OF MODIFIED HYALURONIC ACID
3.3.1. Clinical evaluation of the efficiency and tolerability of modified hyaluronic acid preparations
The results of subjective drug efficiency assessment by patients of different age groups are presented in Table 7. Based on this information, we can note the positive effect of the intradermal injection of modified HA preparations on the principal external signs of involutional skin changes.
Table 7
The results of subjective skin condition assessment by patients after a course of procedures
Criteria
Average subjective score (on a 4-point system: from 0 to 3)
Group 1
Group 2
Group 3
Skin elasticity
2.6
2.5
2.5
Complexion improvement
2.5
2.4
2.6
Reduced wrinkles
2.7
2.5
2.5
Moisturizing effect
2.5
2.5
2.5
According to the results ("Skin elasticity" and Reduced wrinkles” criteria) at the end of the bioreparation course, all patients noted thickening of the skin in areas with reduced elasticity: improvement of face oval, decreased severity of sagging tissues in the central part (the so-called "flews").
Positive effect of the technique was also noted in the perioral region, including smoothing of the nasolabial folds and decreased severity of the tissue depression area in their central part. Wrinkles of the upper lip (mimic and static-dynamic) became less pronounced after the course of therapy.
In the periorbital region, according to the patients, there was a general improvement in tissue condition, manifested in the smoothing of various degrees of radial wrinkles ("crow's feet"). In addition, most of the patients noted thickening of the skin in the the lower eyelids area, manifesting itself in the decrease in the severity of “eye-sacks” and improvement in skin color in this area.
The skin of the lateral areas of the face near the parotid wrinkles and folds (depending on the degree of their severity) also improved according to the patients’ visual assessment.
Patients who had mimic wrinkles of the forehead noted an improvement in the condition of this zone, ranging from leveling to significant smoothing. Generally, there was a tendency for static-dynamic wrinkles to turn into dynamic ones, i.e. all patients noted smoothing of fine wrinkles and a decrease in deep wrinkles, to various degrees.
According to the results of testing ("Complexion improvement” criterion), all patients noted a significant improvement in color and smoothness of skin tone, i.e. reduction of complexion unevenness. As already noted, this effect was also noted in the lower part of the periorbital region, where the severity of under-eye dark circles decreased.
According to the results of testing ("Moisturizing effect” criterion), the therapy improved the patients’ skin condition. Moreover, this effect tended to increase and prolong with the increase of treatment sessions number. The patients also positively characterized the feeling of moisturizing and softness of the skin, i.e. absence or significant reduction of the usual feeling of tightness plus almost no manifestations of skin flaking (in persons with dry skin type) along with a tactile improvement of skin texture.
The overall assessment of the technique was quite high at the end of the therapy. The best responses were obtained in patients aged 40-55 years, who mentioned improvement in skin elasticity, skin tone and complexion smoothness, reduction in the severity of wrinkles and folds, up to smoothing out small and unexpressed skin defects.
Patients aged 35-40 years with moderate signs of photo- and chronoaging noted improvement in the general condition of the skin, including improvement in complexion and decrease in reactivity to various irritating factors.
The older age group (56-62 years) noted improvement in skin tone, decrease in the severity of wrinkles to less pronounced, smoothing of nasolabial folds, as well as in the lower part of the face. The decrease in the severity of the deformation type of aging (“flews”) in this group was noted as “good”, but insufficient.
Negative assessments were associated with the peculiarities of the intradermal injection method (which the patients were warned about in advance). The use of anesthetic application agents before the mesotherapy session somewhat reduced the discomfort from the procedure. The negative effects noted by patients included post-injection hematomas and painfulness during needle injection. The introduction of the drug, the condition of the skin in the area of injections in the post-injection period caused by the presence of the drug, did not bring negative consequences.
It is worth noting the fact that the formation and severity of hematomas following vascular damage during injections decreased as the course of therapy progressed, which may be due to the beneficial effect of hyaluronic acid and the biological substances included in the preparations.
No allergic reactions were noted. There were no episodes of skin peeling, irritation or redness, as well as swelling of the face during the entire study. Some swelling of the drug injection zones was noted during the first, maximum second day, depending on the initial tendency of the tissues to fluid retention. This is due to the hydrophilicity of hyaluronic acid forming the preparation.
Thus, the overall subjective patient assessment of the results of modified HA intradermal injections is positive. The technique was rated by the vast majority of patients as very effective. There were no adverse reactions and complications during and after the end of the course of treatment.
3.3.2. Influence of modified hyaluronic acid injections on skin elasticity
Currently, the elastic properties of the skin are considered as the main markers of its age. A decrease in skin elasticity and firmness is observed during both photoaging and chronological aging due to a decrease in the number and/or degradation of elastin fibers (33, 100).
In the studied age groups, we noted differences in the elastic properties of the skin. In group 1, the indicators of skin elasticity were within the normal range. In group 2, there was a tendency for decreasing indicators with a predominance of reduced values, which amounted to 75.8% of the normal indicators of elasticity (p less than 0.05). In group 3, low values of skin elasticity prevailed – 47.8% of the norm (p less than 0.05).
After the therapy, an improvement in skin elasticity was found in all the groups (Table 8, Fig. 45).
Table 8
Dynamics of average skin elasticity indicators
Mean value (U), M±m, p less than 0.05
Before
After
Group 1
44.3 ± 2.3
47.3 ± 1.5
Group 2
33.6 ± 3.8
44.8 ± 2.1
Group 3
21.2 ± 2.1
27.4 ± 1.3
The analysis of this study showed a positive effect of modified HA preparations on skin elasticity in all age groups. In the youngest patients (group 1), where the elasticity of the skin was normal even before the therapy, the elasticity indices measured after the course slightly increased.
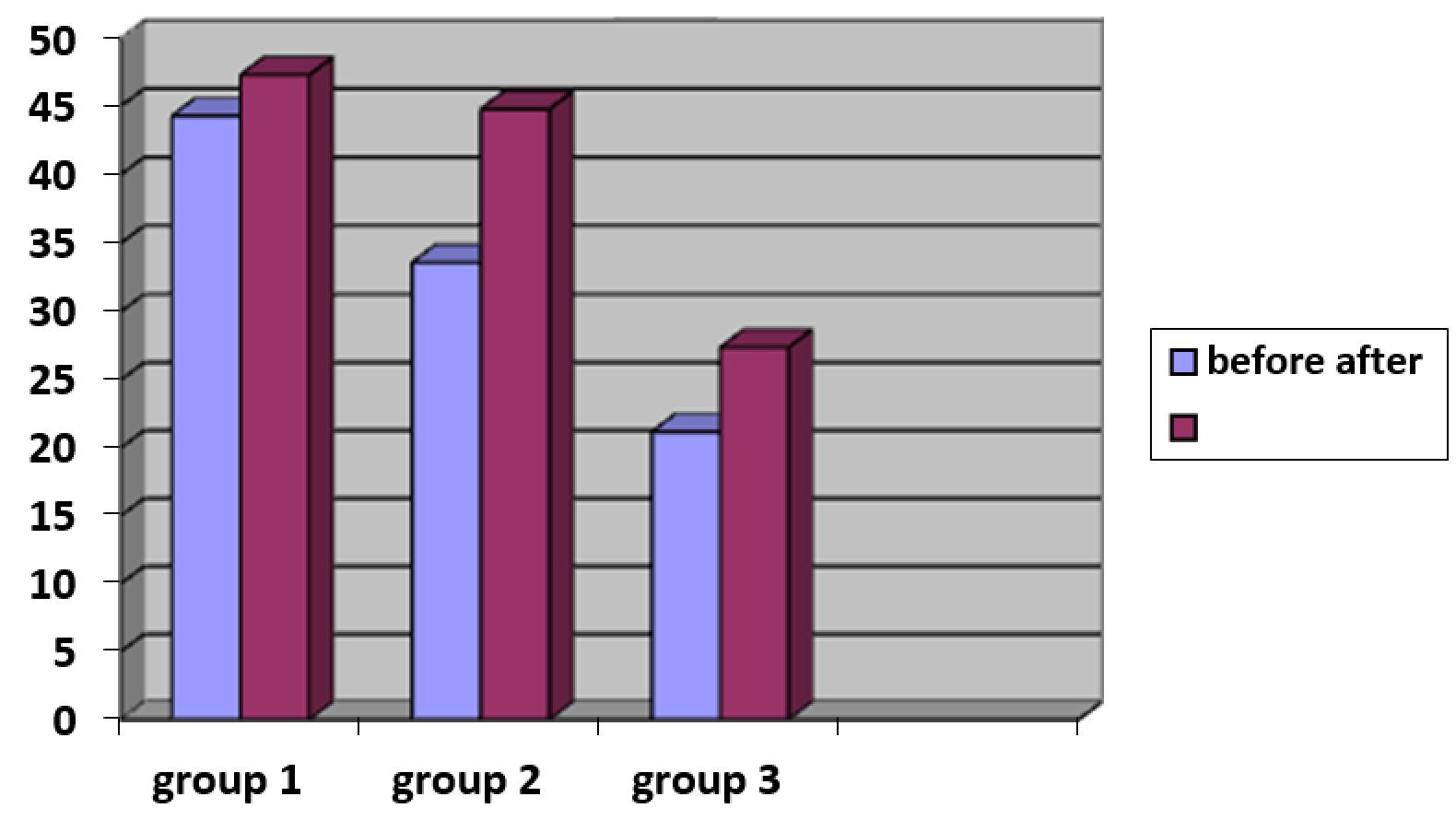
Fig. 45. Dynamics of average elastometry indicators
In group 2, the indicators increased by 1.3 times in relation to the indicators of skin elasticity before therapy (p less than 0.05). In the older age group (group 3), good results were also obtained (an increase of 1.3 times), but, given the low initial elasticity indicators in these patients, the resulting upward trend in indicators was not as pronounced as in other groups, which may be due to the insufficient duration of therapy for these patients.
Thus, the noted changes in the parameters of elasticity and firmness of the skin of patients of different ages after the use of modified HA indicate an improvement in the state of the fibrous structures and strengthening of the dermis' connective tissue framework.
3.3.3. Changes in the skin’s functional characteristics – moisture, pH, and greasiness
The functional characteristics of the skin determine its barrier properties. The most significant indicators to assess the skin’s functional reserve are its pH, oil content and moisture content.
In general, after the course of therapy, the results of skin pH-metry showed positive dynamics. In the first groups, pH values were aligned to the figures corresponding to the physiological values of this parameter. According to the study, in the 3rd observation group, the pH values during the initial study and at the end of the course of therapy were within the normal range, but there was a tendency to some decrease in the values at the end of therapy (Table 9; Fig. 46).
Table 9
Dynamics of average pH values
Skin pH (U), M±m, P less than 0.05
Before
After
Group 1
7.1 ± 1.36
6.1 ± 0.9
Group 2
5.96 ± 1.3
5.6 ± 0.8
Group 3
5.7 ± 1.01
5.54 ± 0.9
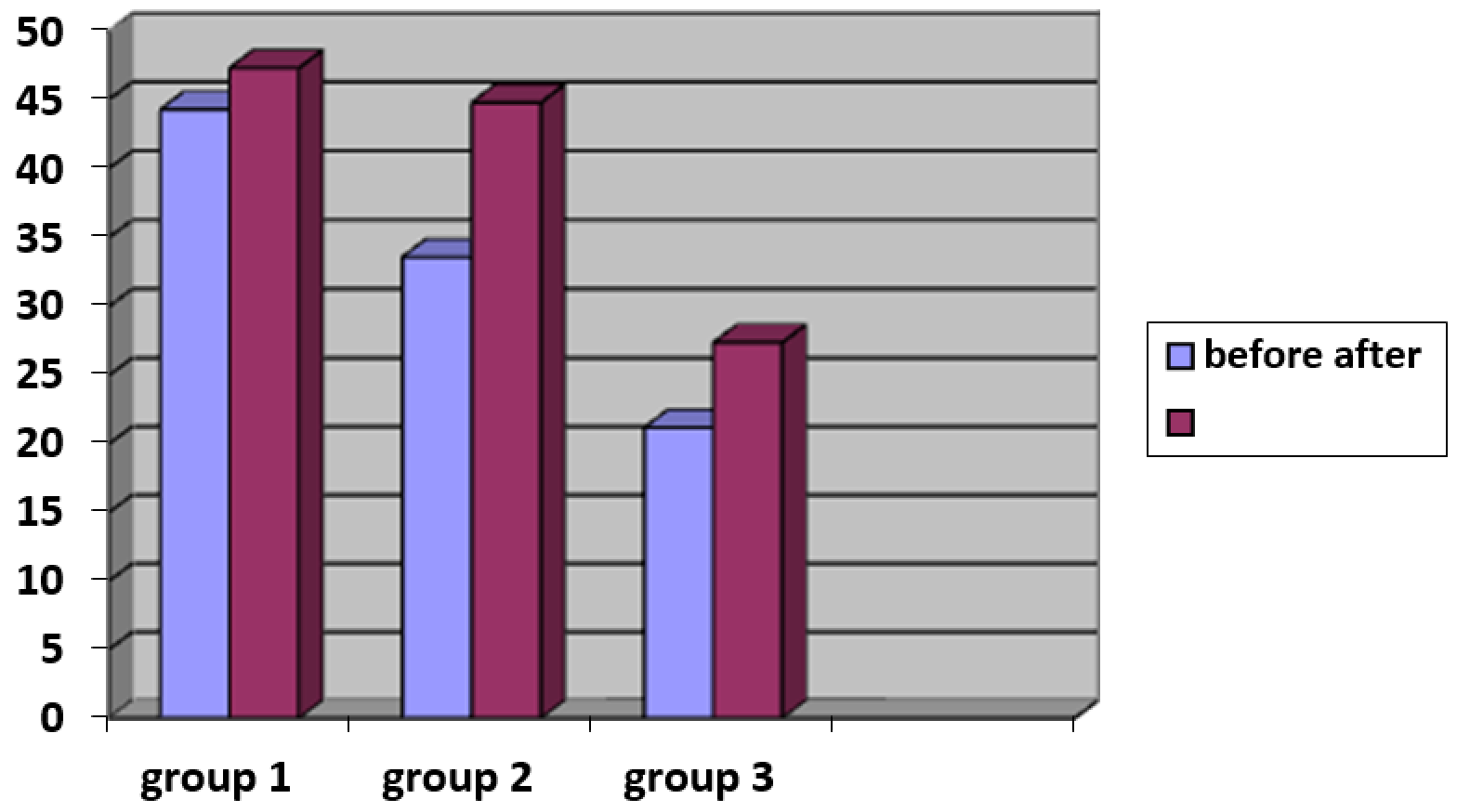
Fig. 46. Dynamics of average pH values
Thus, after a course of modified HA preparations injections, there was a tendency to restore normal physiological indicators of skin acidity, which indicates an improvement of the epidermis’ protective mechanisms. Our results generally correspond to the data available in literature regarding normalization of skin pH values with the use of geroprotectors (antioxidant therapy in the form of Triovit intake) (19).
It has been shown that age-related changes affect skin appendages, including sebaceous glands (99). Sebum production decreases by approximately 23% per decade, which makes a significant contribution to the weakening of the barrier properties of the skin's hydrolipidic barrier (26). In this regard, it was important to evaluate the effect of the introduction of modified HA on skin oil content. Average sebometry data are presented in table 10.
Table 10
Dynamics of average sebometry indicators
Mean value (µg/cm2), M±m, p less than 0.05
Before
After
Group 1
39.8 ± 4.7
44.2 ± 1.34
Group 2
34.3 ± 6.3
40.1 ± 2.1
Group 3
25.7 ± 3.4
37.5 ± 2.2
According to this study, in the 1st observation group, a predominance of normal values of indicators was diagnosed, i.e. the presence of a normal skin type, although some of the patients had low values. In group 2 at the initial study, lower values were obtained in patients with normal and dry skin types, and the proportion of the latter prevailed over the former. In group 3, the overwhelming number of values indicated dry skin type. Only in 3 patients aged 56-57 years had normal skin type according to testing results, which confirms the tendency of aging skin to become dry or normal prone to dryness. In the dynamics of observation, a general trend was determined in all groups towards an increase in sebometric parameters. The best test results before and after the course of therapy were obtained in the 1st observation group. In groups 2 and 3, an improvement in sebum parameters was also noted, i.e. approaching the values corresponding to normal level of sebum production. In group 2, more significant numerical values of indicators were noted than in group 3 at the end of the course of bioreparation (Fig. 47), although in a comparative analysis a greater positive result was obtained in group 3, where the average sebometric index increased by 46% after the course of procedures.
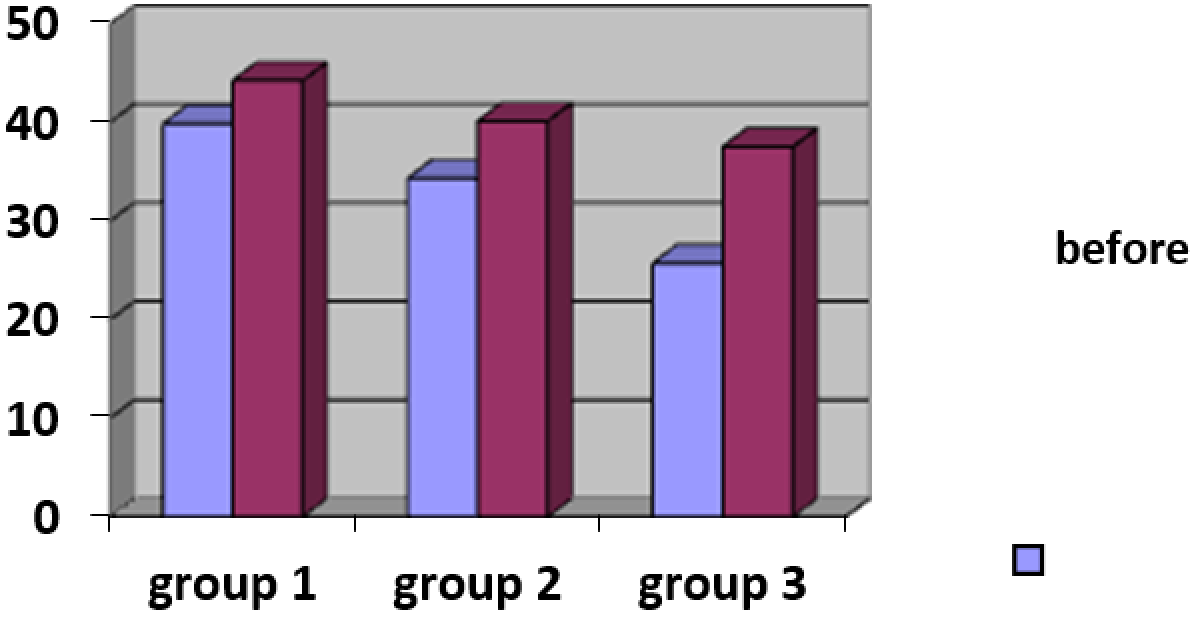
Fig. 47. Dynamics of average sebometry indicators
In the literature studying the effect of intradermal HA injections on the severity of involutive skin changes, we did not find information about the activation of the sebaceous glands function. Apparently, the lipid balance normalization occurs against the background of the beneficial effect of modified HA on the state of metabolic processes in skin, resulting in an improvement of its appendages’ function.
The obtained results of sebometry indicators evaluation correlate with the results of pH testing. The level of oil content and the pH value of the skin are directly interdependent, and at the end of the course, they improved in all groups, which indicates the normalization of the morphofunctional state of the epidermis.
It has been shown that skin hydration also decreases with age because of decreased intensity of microcirculation, impaired water-retaining properties of the dermis, and decreased barrier functions of the skin. In this regard, the effect of intradermal administration of modified HA preparations on skin hydration was evaluated.
An analysis of the starting moisture values showed a general trend towards a decrease in the level of hydration. In group 1, the predominance of moisture values corresponding to the average level of tissue hydration was revealed. In group 2, there was a tendency to reduced level of moisture: test data range from average values to the lower limit of the norm. In group 3, indicators corresponding to low values of moisture prevailed. The results obtained were consistent with the available literature data on skin dehydration that develops with age (15, 28).
After the therapy, an increase in moisture indicators was noted in all three groups (Table 11, Fig. 48).
Table 11
Dynamics of average skin hydration indicators
Mean value (U), M±m, p less than 0.05
Before
After
Group 1
63.2 ± 8.3
73.2 ± 8.1
Group 2
48.9 ± 13.0
60.9 ± 6.5
Group 3
24.3 ± 6.5
45.5 ± 7.3
The results were best in group 1 and good in group 2. In group 3, there was also an improvement in skin hydration, but the maximum values in this group 20 days after the end of the course of therapy were at the level of medium-low values. When comparing the increase in average skin hydration after a course of procedures in different groups, it was found that in the older age group the difference was the most significant: the degree of moisture had increased by 87%.
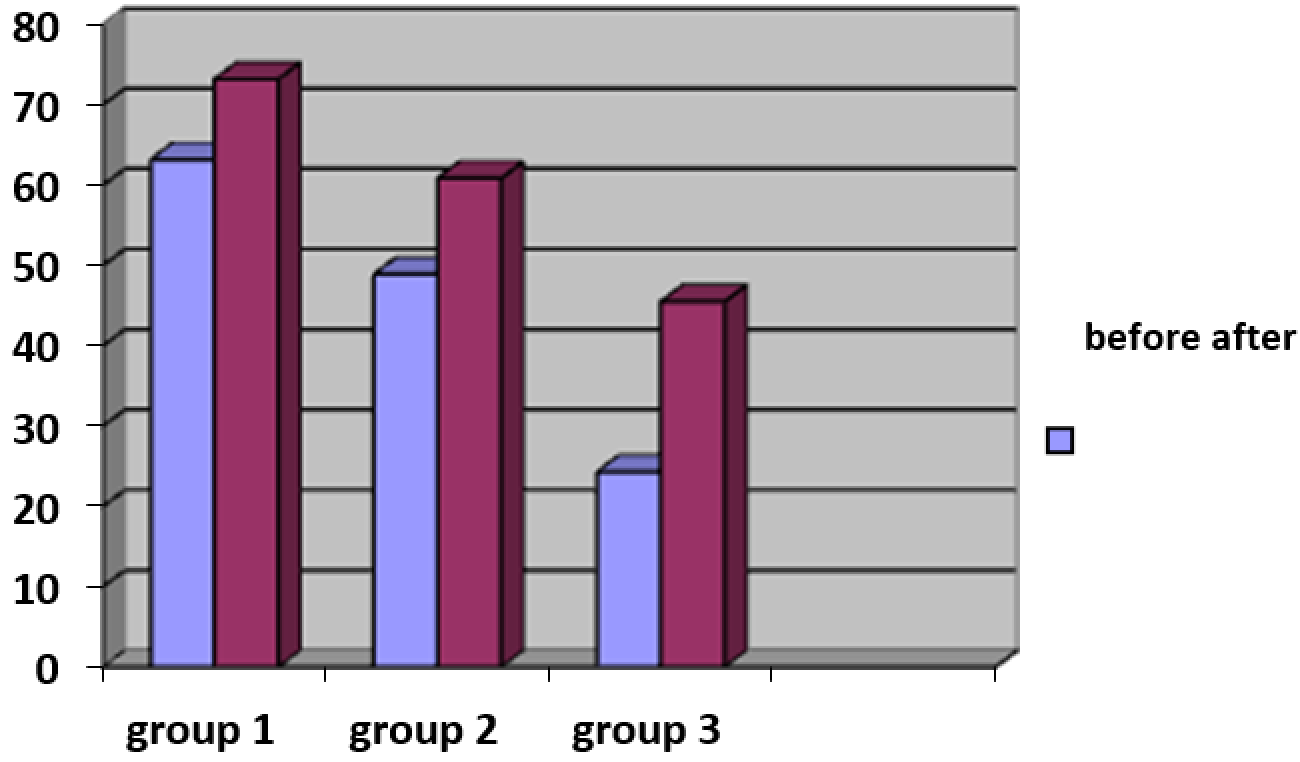
Fig. 48. Dynamics of average corneometry indicators
Thus, the general tendency to improved hydration indices of the surface layers of the skin confirms the effectiveness of the modified HA injection technique. For skin moisture and oiliness values, the most significant increase after the therapy took place in the older age group. But, given the lowest initial values of these parameters, their maximum values after therapy still remained within the range of low and medium values. Perhaps in group 3 (age limit 56-62 years), the course of modified HA therapy should be longer (more than 3 procedures) for optimal compensation of age-related deficiency of tissue hydration and disorders of the skin's lipid barrier.
3.3.4. Evaluation of change in skin microtopography by ultrasound scanning
Ultrasound scanning was used to assess the condition of the epidermis (uniformity, discontinuity of this layer) and dermis (quality of subepidermal layer, uniformity of structures in this area).
The study revealed differences in skin structure in patients of different age groups.
As a rule, in group 1, the epidermis was represented by a moderately thickened bright line with a small number of thinning areas. The papillary layer of the dermis had moderate manifestations of rarefaction, the reticular layer had relatively preserved distribution of dense structures.
In group 2, more significant changes were noted at the level of the epidermis – lesser thickness, greater degree of line discontinuity. The subepidermal space had manifestations of rarefaction with some evenly distributed blackout areas. The deeper layers of the dermis were distinguished by a lower degree of density. The total skin thickness tended to be smaller than in group 1.
In group 3, the epidermis had the greatest changes – smaller thickness with large areas of interruption. The papillary and reticular dermis had a lower density, and the greatest changes were noted in the subepidermal space.
After the therapy, the indicators according to the ultrasound scan had a positive trend (Table 12). In general, a more uniform structure of the epidermis was noted with a decrease in thinning areas, evener fit to the dermal structures, which, along with the alignment of its contours, indicates the restoration of the morphofunctional state of this layer. From the side of the epidermal-dermal layer, there was an increase in thickness, evener distribution of structures, i.e. an increase in hyperechogenicity and a reduction in areas of hypoechogenicity, including thickening of the papillary dermis, which may indicate an optimization and improvement in the functional state of the skin, reflecting the synergism of the drug components’ biological action.
Table 12
Dynamics of average ultrasound values of the skin
Average epidermal-dermal thickness, mm
Before
After
Intensity (density) of dermal structures, %
Before
After
Group 1
2.1 ± 0.1
2.33 ± 0.16
4.3 ± 0.35
5.7 ± 0.5
Group 2
1.65 ± 0.3
1.9 ± 0.14
4.0 ± 0.45
5.7 ± 0.25
Group 3
1.56 ± 0.21
1.7 ± 0.21
3.6 ± 0.22
4.7 ± 0.3
In group 1, after therapy, there was an improvement in the ultrasound picture of the skin. The epidermis tended to have a structure more uniform in thickness with less thinning areas, better brightness of the layer, which can be regarded as sign of thickening of this layer. The structure of the dermis was a layer with a more uniform density and significantly less darkening areas, as well as an increase in number and uniformity of high density areas distribution, i.e. thickening of this layer of skin was noted. The data obtained are presented on the example of a patient included in this group (Fig. 49).
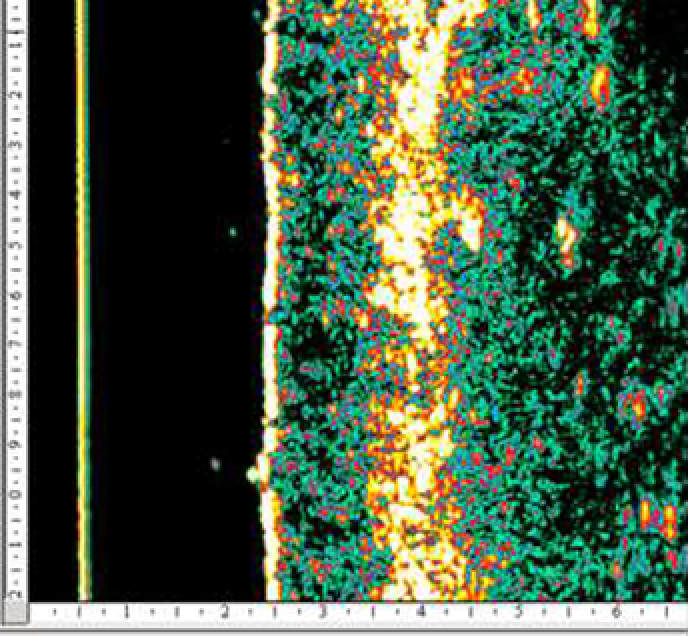
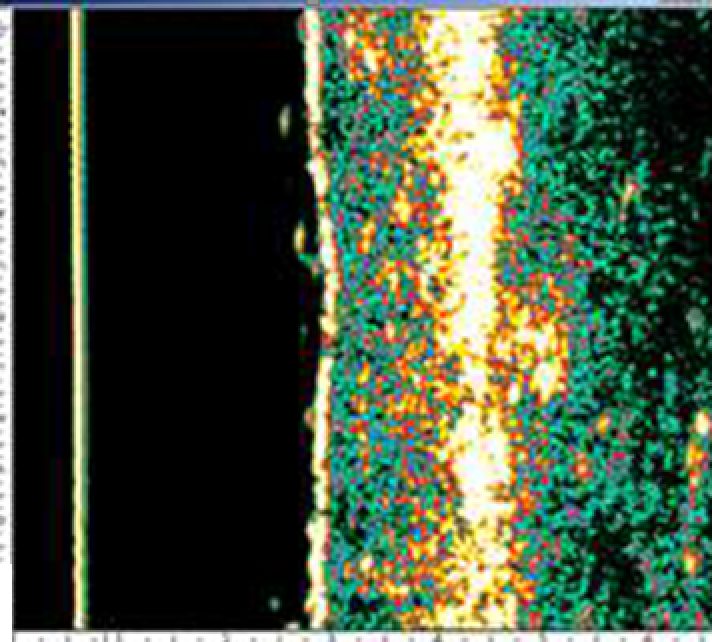
Fig. 49. Patient O., 35 years old. Dynamics of the ultrasound picture of the skin. A - before, B - after the course of modified HA therapy.
In group 2, changes were most clearly visible in the subepidermal region, with a significant increase in layer’s density with more uniform distribution of low and high density areas. The reticular dermis also tended to increase in density given an increase in the number of high-density areas. The epidermis after the course of bioreparation had a more uniform structure (Fig. 50).
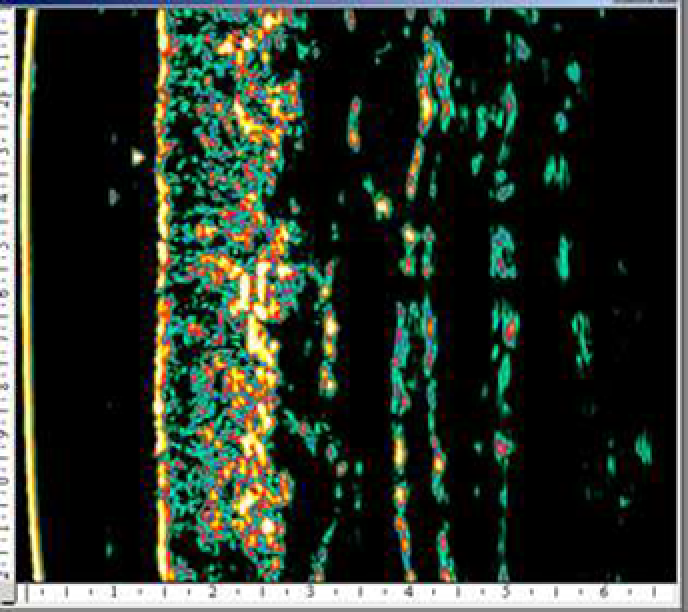
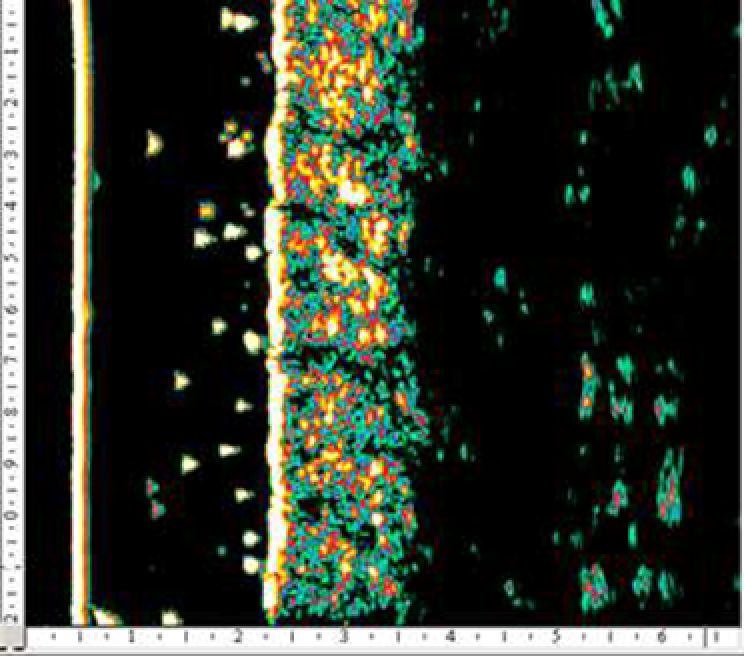
Fig. 50. Patient R., 43 year old. Dynamics of the ultrasound picture of the skin. A - before, B - after the course of modified HA therapy.
In group 3, there was a general trend towards improvement in skin picture. From the side of the subepidermal space, after the course of therapy, a significant decrease in rarefaction was noted due to increased echogenicity of this layer, which corresponds to the high biological activity of the drug-modified HA components. The density and uniformity of epidermis and dermis structures were typical for this group (Fig. 51).
Fig. 51. Patient M., 60 years old. Dynamics of the ultrasound picture of the skin. A - before, B - after the course of modified HA therapy.
The obtained ultrasound scanning results indicate an improvement in the skin's morphofunctional state in 3 age groups of observation, including a diagnostically important zone, the subepidermal space, which reflects the main involutional changes in the skin during photo- and chronoaging.
3.3.5. Evaluation of skin microrelief changes
Visioscanning was used to assess the relief indicators. Positive dynamics was noted for all studied indicators (Table 4.3.2.1.):
• SEsc - scaliness;
• SEr – roughness;
• SEsm - smoothing;
• SEw - wrinkles.
Table 13
Dynamics of average skin microrelief parameters
Mean value (U), M±m, p<0.05
SEsc (scaliness)
SEr (roughness)
SEsm (smoothing)
SЕw (wrinkles)
Before
After
Before
After
Group 1
2.1 ± 0.1
2.33 ± 0.16
4.3 ± 0.35
5.7 ± 0.5
Group 2
1.65 ± 0.3
1.9 ± 0.14
4.0 ± 0.45
5.7 ± 0.25
Group 3
1.56 ± 0.21
1.7 ± 0.21
3.6 ± 0.22
4.7 ± 0.3
By analyzing the data obtained, an improvement in skin parameters was noted in all observation groups. The best results after the course of therapy were obtained in group 1, where the patients initially had less pronounced changes compared to other groups. So, in this group, for the purposes of this study, moderate values of scaliness, changes in microrelief and the severity of wrinkles were noted, which, after treatment with modified HA, tended to relative improvement, which might be due to a certain degree of severity of age-related changes in the skin and their morphofunctional compensation at the start of the study.
In group 2, there was also positive value dynamics so they approached the average microrelief parameters of group 1. At the same time, in the age group from 41 to 55 years, the best results were obtained from the use of the drug in all scanning parameters. It is possible that the data obtained are related to the peculiarities of tissue repair in this age group, namely, the preservation of structural restoring mechanisms, enhanced by a complex of biologically active components that make up the preparation.
In group 3, positive dynamics of all indicators was also noted, but the best results among all the studied indicators were obtained when testing the “smoothness” parameter. It increased by 81% in relation to the initial value (p less than 0.05) and approached the values of "smoothness" in patients from group 1. This may be due to the high moisturizing power of the modified hyaluronic acid preparation. The positive dynamics of the parameters “scaliness”, “roughness” and “wrinkles” indicates a beneficial effect of the therapy on the skin in this age group, improved microrelief thanks to reduced depth of wrinkles and folds, smoothing fine wrinkles and compensating for dehydration phenomena.
The analysis of visioscanning results for all groups as a whole showed a positive effect of the employed technique on skin microrelief.
3.3.6. Evaluation of skin microtopography by confocal laser scanning microscopy
The study of confocal laser scanning microscopy (CLSM) made it possible to analyze the main CLSM signs of involutional skin changes.
At the level of the stratum corneum in all patients, along with the characteristic non-nuclear polygonal cells, highly refractory white inclusions (scales) were determined, the number of which corresponded to the severity of skin xerosis (Fig. 52).


Fig. 52. CLSM – corneal layer picture: A – patient N., 38 years old, clinically normal skin type with minimal signs of dehydration B – patient N., 58 years old, clinically dry skin type with moderate manifestations of dehydration
With further deepening of the scanning, relative preservation of subsequent layers’ normal architectonics was noted with the visualization of cells with characteristic histological features.
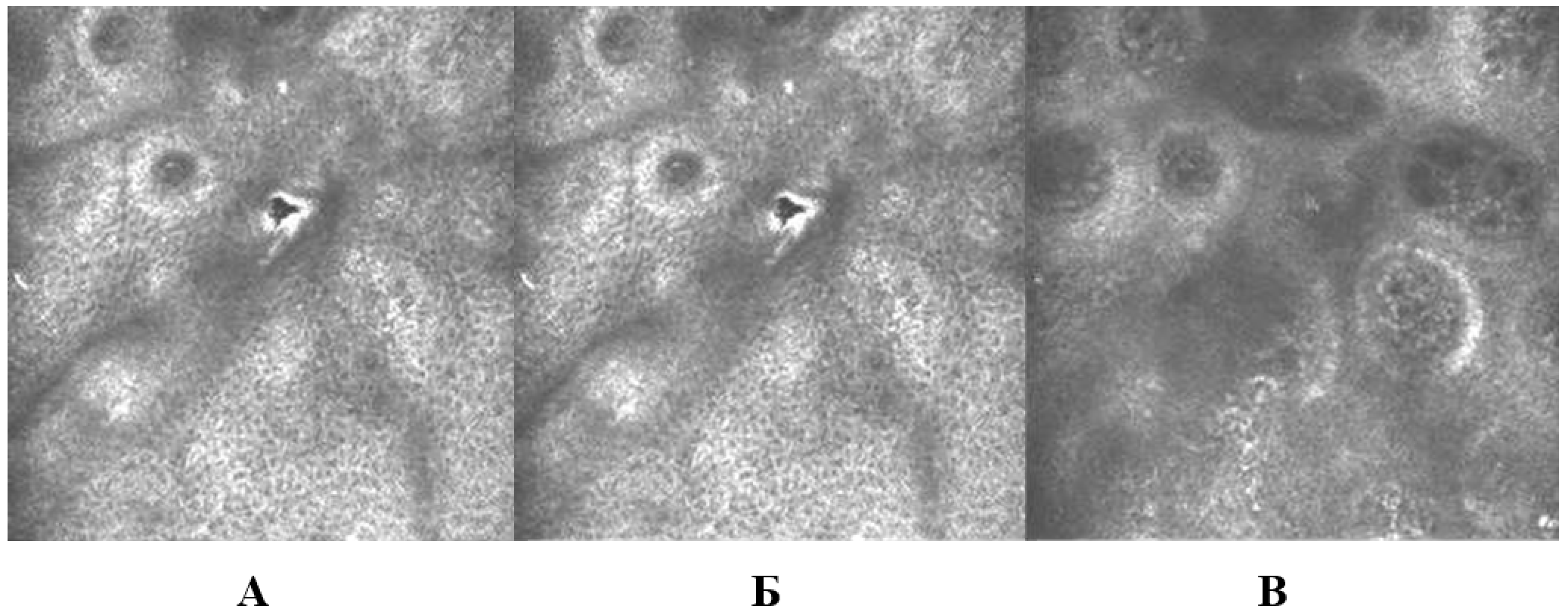
Fig. 53. CLSM – picture of the epidermal junction; dynamics as the scanning deepens: A – 29 microns; Б – 49 microns; B – 69 microns.
Above the level of the upper part of the basement membrane, depending on the patient’s phototype, there was a different degree of saturation of the section with highly refractive, i.e. having a white color, rounded cellular structures, the so-called "melanin caps", corresponding to a section at the level of the tops of the dermal papillae (Fig. 53).
With further scanning, the cross section of the papillae of the dermis became blurry, fuzzy, there was a loss of a clear rounded and ovoid shape. As it deepened, there was a decrease in the distance from the tops to the base of the papillae, which corresponds to a decrease in the height of the papillary dermis. At the same time, there was also a tendency to a larger diameter of the papillae with the severity of skin involution, which may indicate smoothing of the papillae of the dermis. In older patients, during the visualization of skin structures at the level of the upper layers of the dermis, fixation of papillary capillaries was noted in a smaller number than in younger subjects.
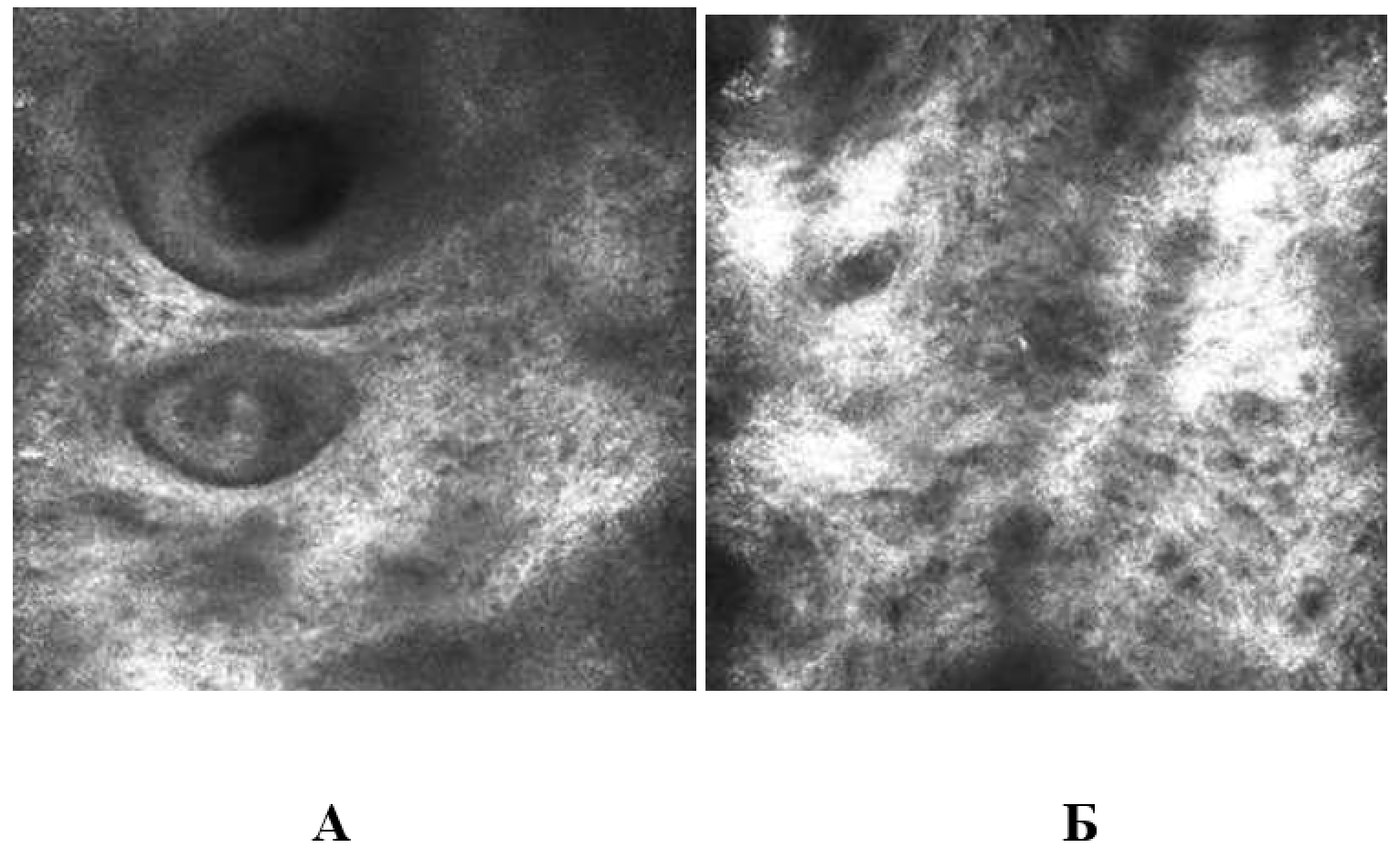
Fig. 54. CLSM – papillary layer picture: A – patient N., 34 years old, scan level 64 µm; B – patient M., 60 years old, scan level 64 µm
Depending on the thickness of the stratum corneum and the severity of xerosis manifestations, which affect the degree of penetration and reflection of laser radiation in the tissues, in the deeper layers of the dermis there were areas with intersecting fibrous structures of unequal thickness with varying degrees of architectural chaos, which reflects the severity of the dermis intercell frame degradation (Fig. 54).
The general trend of CLSM skin pictures after 3 procedures of the introduction of modified HA can be described as general improvement in the degree of layer refractoriness, i.e. improving the optical properties of the tissue, which allows for exploring the deeper layers of the scan. This property may be due to the restoration of the level of hydration and the optimization of tissue hydroreserve mechanisms.
Layer-by-layer scanning of the epidermis revealed the preservation of the cellular composition of each layer and the normal relative position of all structures.
In group 1, according to the CLSM data, there was a complete leveling of the manifestations of xerosis (a decrease until the complete disappearance of white brightly colored polygonal structures on the surface of the stratum corneum – scales). The structure of the epidermis layers and the cellular composition were visualized with a greater degree of clarity, which is due to the normalization of the tissue’s refractory properties.
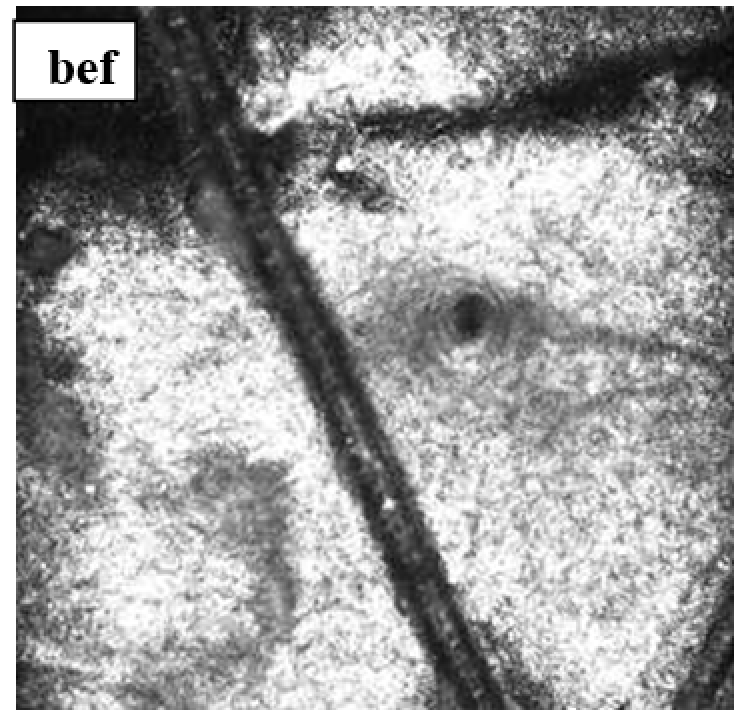
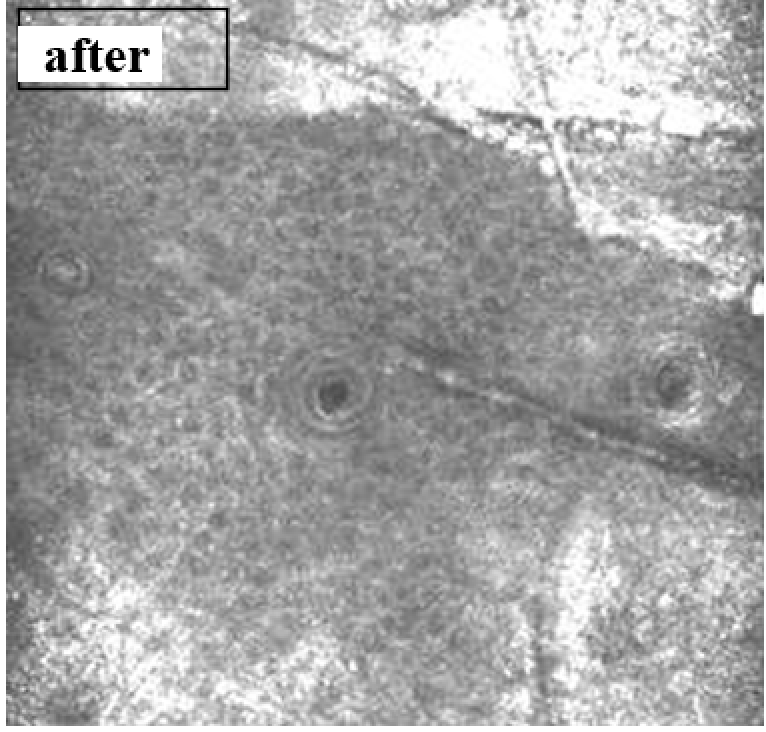
stratum corneum (scanning level 0 µm)


epidermis-dermis junction (scanning level 49 µm)
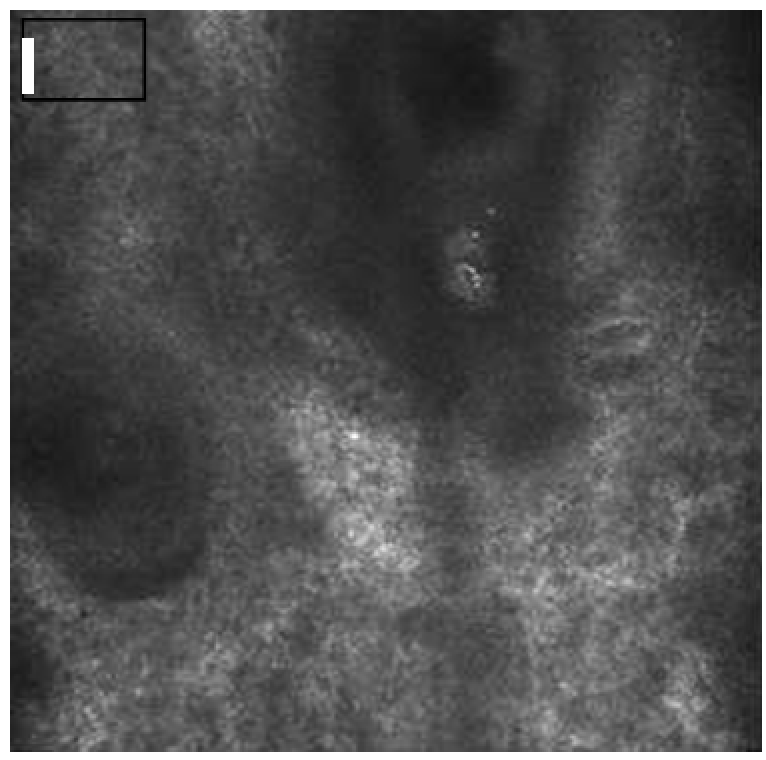

reticular dermis (scanning level 79 µm)
Fig. 55. Patient N., 34 years old, dynamics of CLSM during a course of therapy with a modified HA preparation.
According to CLSM data, dermal structures over time showed better refractoriness with greater scanning depth. The architectonics of the skin's dermal layer did not undergo significant changes, which corresponds to the age-related morphological features of aging changes for this group (Fig. 55).
stratum corneum (scanning level 0 µm)
epidermis-dermis junction (scanning level 39 µm)
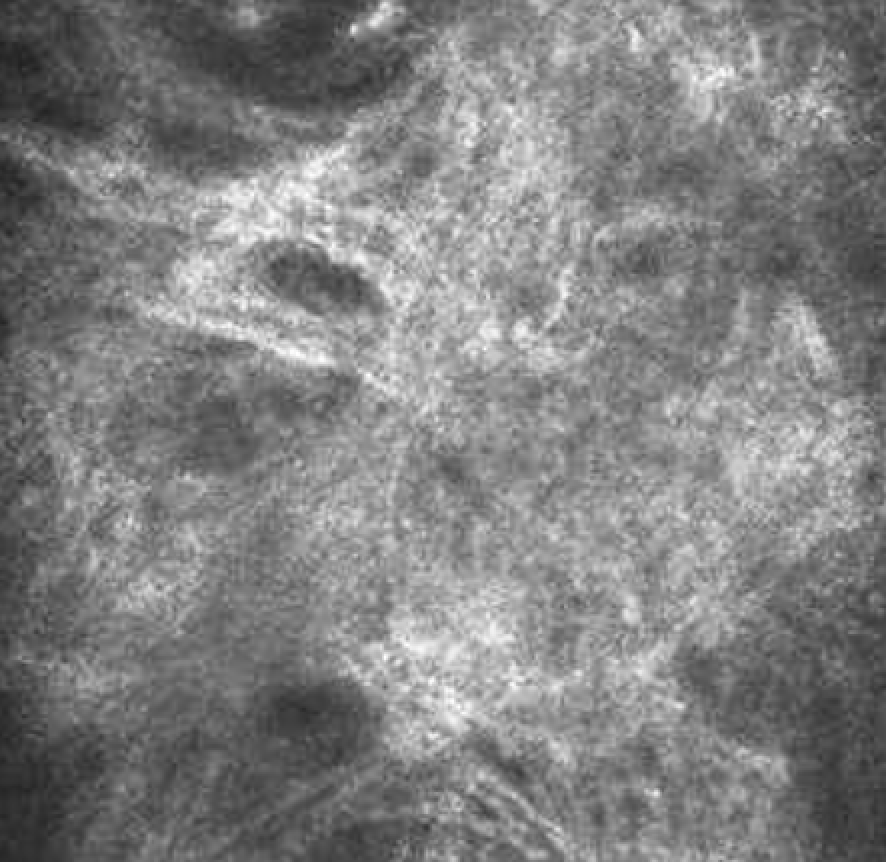
reticular dermis (scanning level 69 µm)
Fig. 56. Patient O., 49 years old, dynamics of CLSM during a course of therapy with a modified HA preparation.
In group 2, initially there were more pronounced manifestations of dry skin, a greater degree of refractoriness of structures at all levels of scanning. Dermal structures were characterized by moderate or middle degree of architectural chaos, represented by uneven thickening of the fibers, the presence of moderate amounts of monomorphic structure areas. The structure of the epidermis and the cellular structure of each layer according to the CLSM data after the course of therapy retained their morphological features without a tendency to any changes, which indicates the safety of the technique.
The overall refractoriness of tissues in group 2 also tended to improve, which was manifested in better visualization of the deeper layers of the scan.
The dermal fibers had a more delicate structure with a tendency towards a more uniform reticulate organization. The pronouncedness of the monomorphic structure areas was less noticeable, but they also did not disappear completely (Fig. 56).
Group 3 showed the most pronounced changes at all scan levels. The stratum corneum had all the signs of age-related changes: thickening of stratum corneum, presence of abundant scales on the surface, more exaggerated dermatoglyphics.
There was a tendency to decreased thickness of the epidermal layer with a preserved cellular composition, but with individual areas interspersed with more intense staining, i.e. characteristic age-related focal hyperpigmentation.
When scanning the level of the dermal layer, a deterioration in the quality of visualization of structures was noted – less clarity and the need to maximize the contrast of images, which indicates poor refractoriness of the tissues under study. Fiber structures had a large thickness unevenness along the length, distribution in the form of a chaotic network structure with an abundance of monomorphic areas. Figure 57 shows the results of comparative observations.
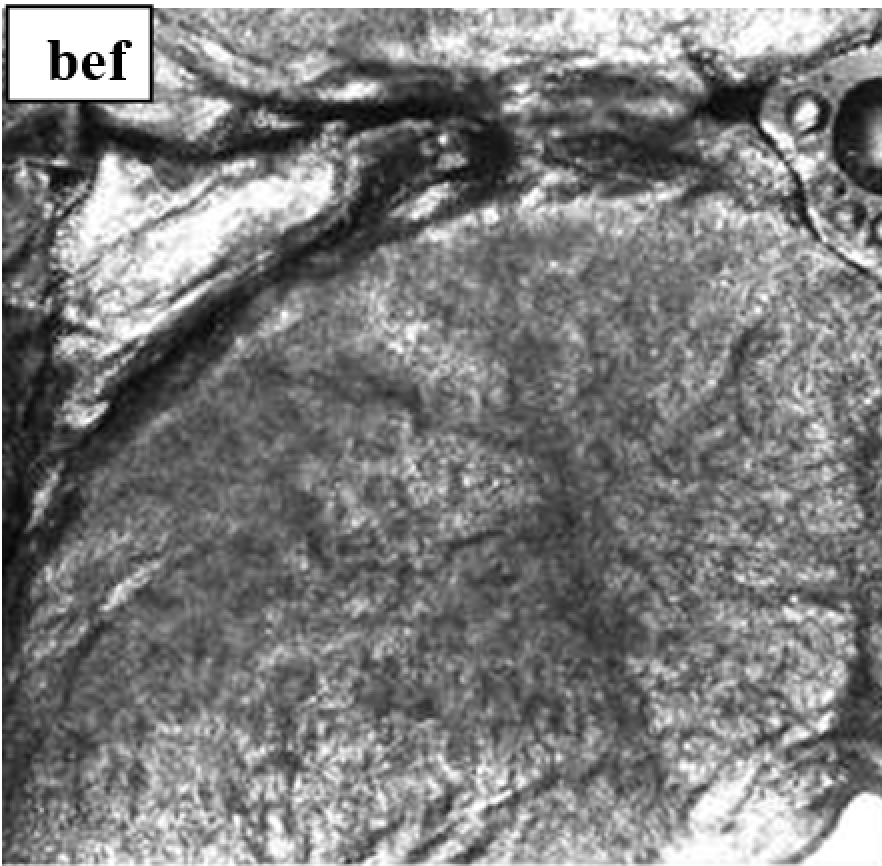

stratum corneum (scanning level 0 µm)
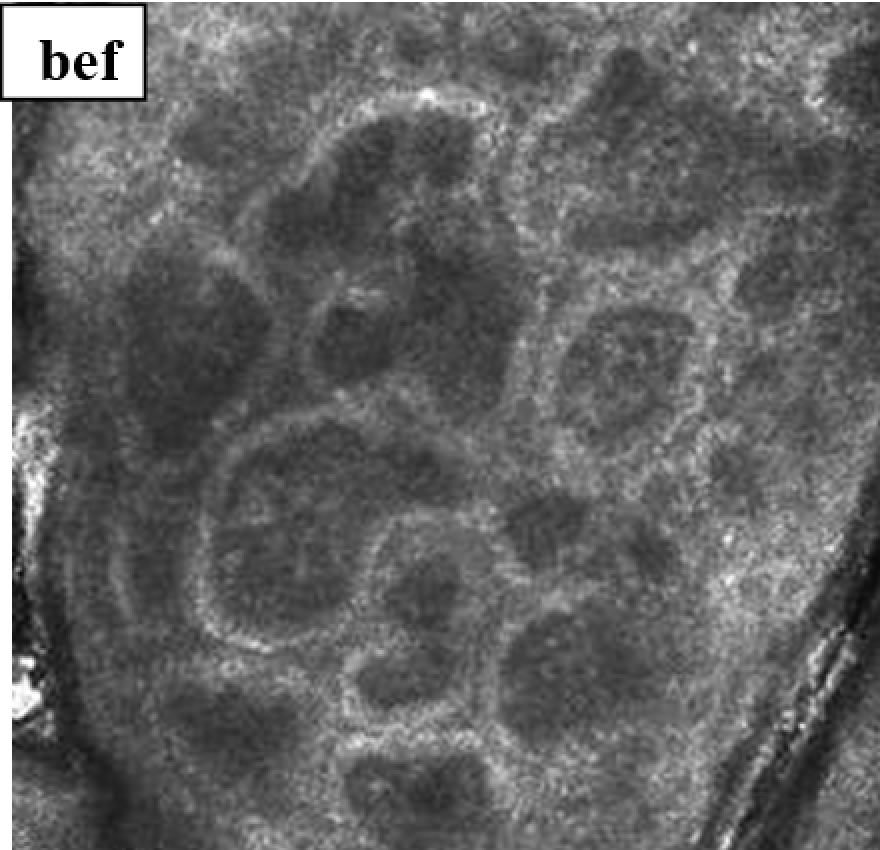
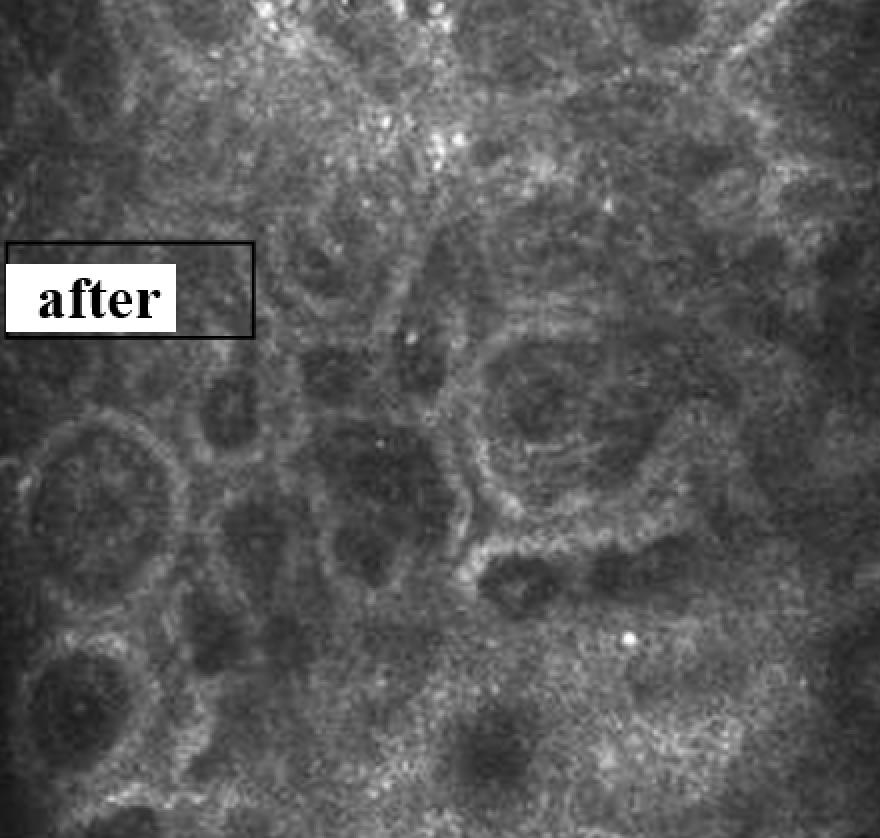
epidermis-dermis junction (scanning level 44 µm)

reticular dermis (scanning level 64 µm)
Fig. 57. Patient M., 60 years old, dynamics of CLSM during a course of therapy with a modified HA preparation.
After modified HA injections, xerosis of the surface layers of the skin significantly decreased; the structure of the surface layer (“starting layer”) became more uniform, and the number of scales notably decreased. As in other groups, the structure, quantitative and qualitative composition of the epidermal layers of the skin did not undergo any pronounced changes, which is indicative of good adaptation of age-related tissues to injection methods using HA.
At the level of the dermis, improvement in the quality characteristics of the mesh structure of this layer was noted. The fibers had a more orderly arrangement with a tendency to more uniform structure. The quantity and density of the sections of the monomorphic structure also had dynamics to decrease.
For an objective assessment of the data of CLSM scanning, the parameters of the average epidermis thickness, corresponding to the rehabilitating effect on this layer of the skin, were studied. The state of the epidermis also indirectly reflects the preservation of the mechanisms for creating and compensating for changes in the hydroreserve and metabolic activity of the skin's deeper layers. Thus, when analyzing the obtained data, positive changes were understood as restoration of the level of moisture, optimization of the processes of synthesis of hydrophilic components, normalization of the processes of keratinization and cell proliferation. Technically, the numerical value of this parameter was equal to the distance from the surface of the stratum corneum to the level where lower sections of the dermal rings turn into dermis structures. The results obtained are presented in Table 14.
According to the data obtained, there is an improvement in the studied parameter in all 3 observation groups. The best results were obtained in groups 1 and 2, where, along with an increase in the layer thickness, there is an improvement in tissue refractoriness at all scanning levels, positive dynamics of dermal structures in the form of a more uniform arrangement structure on deep layers with a more measured fiber thickness.
Table 14
Dynamics of CLSM values
Average thickness of the epidermis, microns
Before
After
Group 1
83.52 ± 3.4
88.5 ± 4.3
Group 2
74.3 ± 5.7
79.7 ± 3.8
Group 3
64.5 ± 4.6
72.1 ± 3.5
In group 3, the response of the epidermal layer was also obtained in the form of thickness increase. In general, this group showed the greatest manifestations of compensation for existing skin xerosis, as the most pronounced symptom complex among all the observed patients. Positive changes at the level of the reticular layer in this group were less pronounced than in other groups.
CONCLUSION
The published data that have appeared in recent years have significantly expanded the understanding of the role of HA for the skin. It is considered not only as passive filler of the intercellular space, but also as the natural regulator of a number of biological processes (migration and proliferation of fibroblasts and epithelial cells, stimulation of angiogenesis, activation of homeostasis components, etc.), and in recent years, also as an effective drug delivery and controlled release agent (74).
HA preparations for injection are widely used in the treatment of involutional skin changes. However, literature has practically no studies on clinical efficacy and safety of the injection use of HA preparations. The available data is in most cases not conclusive. Many of them do not give definitive results. There are only a few works in the literature comparing experimental histological studies and clinical observations (26, 35, 79, 85, 86, 108). There are practically no works describing comparative evaluation of the effectiveness of different drugs and methods of their administration. In scientific and practical literature, there is no substantiation of standards for the use of drugs and recommendations for use based on the results of studies.
We have conducted experimental and clinical studies of HA preparations modified with bioactive components. This method of modifying HA is fundamentally new. This is solid-phase modification of HA with bioactive compounds (vitamins, amino acids, oligopeptides), which does not use acids and alkalis as solvents, thereby increasing the environmental friendliness of biopolymer modification processes. The modification of the polysaccharide with various bioactive compounds is carried out simultaneously with the mixing of the two main components in solid state, using increased pressure and shear deformation, which ensures not only their combination on a nanoscale level but also various chemical interactions in the absence of a liquid dispersion medium. This makes it possible to graft water-insoluble compounds (folic acid) onto the HA molecule, and not to change their spatial structure of amino acids and peptides when working with them. As a result, the drugs have a complex effect on involutional skin changes, due to the action of both HA itself and bioactive regulators, which retain their functional activity in the skin and, being gradually released from the drug, stay at the injection site for a long time.
The research included experimental macroscopic, histological, histochemical and electron microscopic studies of the evolution of HA gels modified with vitamins, amino acids and oligopeptides, for subcutaneous and intradermal administration, tissue response to gel injection, as well as a clinical study of the effect of these drugs on involutional skin changes in patients of different age groups.
Macroscopic, histological and histochemical studies of subcutaneous administration of HA gels found that after 1 day, all types of modified HA gels formed single conglomerates under the skin at injection sites, while partially impregnating the nearby subcutaneous fat and connective tissue. Already from the first day, the structure of the gels, consisting of short fibers, toneofibrillar and fine-grained elements, sharply loosens due to watering, since HA is an actively hydrophilic substance. Only in gels modified with vitamin C alone or in complex with amino acids (proline, glycine and lysine), dense condensed gel areas remain, which indicates their denser structure.
By 3-7 days, the impregnation of the surrounding tissues by the gel increases, and the volume of the free-lying gel decreases due to enzymatic hydrolysis of HA and phagocytosis by macrophages. By day 14, gel residues were found in the form of separate small fragments in tissues and/or in hyperplastic lymph nodes and in macrophages that phagocytized the gel.
Depending on the substance immobilized with HA, which determines the physicochemical and biological properties of the drug, the gel was either more rapidly excreted through the lymphatic system or remained longer in the fiber in the form of separate loose conglomerates. An exception was unmodified (native) HA gel, which underwent almost complete resorption as early as on the 3rd day after administration, leaving very small foci of the gel in the cellulose. This was probably due to a lower structuring degree of unmodified HA. A greater degree of gel impregnation of adipose tissue was also noted for it, which was associated not only with a lower degree of molecular structuring but also with high liquid content in the gel.
The dynamics of resorption of the studied gels of modified HA, especially when modified with vitamin C in complex with amino acids, proves a sufficient duration of their presence in tissues, which is a very important positive property because it allows the introduction of drugs to patients in order to correct age-related changes to create a depot of biologically active substances. Long-term presence of HA preparations in tissues is noted in the literature only for stabilized preparations that are used for augmentation and, as a rule, do not have a stimulating effect on the skin.
We noted the development of moderate aseptic inflammation at the border between gels and the surrounding tissues. A moderate neutrophilic infiltration was observed with a maximum on day 1, replaced by lymphocytic-macrophage infiltration at a later date, which could be a manifestation of the tissue reaction to damage through the injection and administration of the drug. By day 14, the content of phagocytic cells and lymphocytes returned to normal in all the studied samples. In none of the cases did a connective tissue capsule form around the implanted gels.
It has now been proven that the invasiveness of the injections themselves provokes processes similar to those occurring during skin wounds, but with less depth and on a smaller scale (3). Damage to various cell types in the epidermis and dermis results in the release of lysosomes and lysosomal enzymes into the extracellular matrix. A cascade of intercellular interactions is launched, consisting of a series of reactions among various types of cells (neutrophils, macrophages, lymphocytes and mast cells) in the damaged tissue under the control of local mediators, growth factors. On the one hand, the processes of cleavage by hyaluronidase of injected and endogenous biopolymers, in particular implanted HA, cleavage of sulfated glycosaminoglycans from the protein part of proteoglycans, proteolysis of proteins, etc., are intensified. On the other hand, the processes of reparative regeneration develop, primarily the proliferation of fibroblasts and the synthesis of collagen by them, which resist destruction and are aimed at restoring the structure of the intercellular matrix. Ultimately, according to the law of excess compensation, reparative processes in tissues not only restore damage, but also lead to renewal (“rejuvenation”) of cell structures and extracellular substance and contribute to reaching a higher stationary level, where balanced synthesis-decay processes occur at an increased rate. HA modified with low molecular weight bioregulators is a source of biologically active compounds necessary for the biorepair processes.
The results of experimental studies (series with subcutaneous injection) confirmed the ability of modified HA preparations to enhance tissue regeneration. At the border between the gel and adipose tissue, the proliferation of fibroblasts and new formation of capillaries gradually increased, starting from the 3rd day of the experiment. These processes were most pronounced with the introduction of the HA preparation modified with vitamin C alone or with amino acids.
Intradermal administration of HA preparations modified with antioxidant and geroprotective substances (vitamin C, glycine, proline, lysine, and vitamin C with glutathione) led to gradual lysis of gels (2nd series of experiments on minipigs), faster than with the introduction of native (unmodified) HA, as well as to pronounced proliferation of fibroblasts and neoangiogenesis. Activation of the proliferative activity of cells was accompanied by an increase in the synthesis and fibrillogenesis of collagen and elastic fibers, which is confirmed by electron microscopy results.
In the discussion of the safety of HA preparations, the question of the development of fibrosis at the site of microimplant insertion is raised (81). Despite attempts to prove the positive role played by the connective tissue capsule at the injection site, in particular around a synthetic filler, it is obvious that the development of fibrosis at the injection site is the body’s defense reaction to a foreign substance and can be considered as a pathological condition that can induce further pathological change.
Histological studies showed that with the introduction of all the studied samples of modified HA, fibroblasts and capillaries grew into the gel, however, at all periods of the experiment (with subcutaneous and intradermal administration), no signs of the formation of a connective tissue capsule around the implant were found. This confirms the high bioinertness and biocompatibility of the injected drugs with living tissues and is a distinctive feature of all the studied samples.
A comparative study of different samples of HA hydrogels showed the specificity of the tissue response to low molecular weight regulators. The studies used preparations of HA modified with vitamin C, folic acid, amino acids (glycine, proline, lysine) and glutathione. It has been established that these low molecular weight substances have geroprotective properties, being antioxidant protection factors, antihypoxants, and also improving skin metabolism. Among them, HA preparations modified with vitamin C and amino acids had a more pronounced effect on skin regeneration. Compared to other samples, the unmodified HA hydrogel did not show such a significant effect on the skin structure.
The results of the experiment were confirmed by the findings of clinical studies. In order to assess the functional and structural changes in the skin after the administration of HA modified with vitamin C in combination with amino acids, 116 patients were examined. In accordance with the severity of age-related skin changes according to the Glogau scale, the patients were divided into 3 groups:
1. from 35 to 40 years old,
2. from 41 to 55 years old,
3. from 56 to 62 years old.
Most of the subjects with involutional changes in the skin and its appendages subjectively noted a significant positive effect: improvement in elasticity, skin color, decrease in the severity of wrinkles, and increase in skin hydration. The latter effect tended to increase and prolong as the number of procedures increased. There were no adverse events or complications.
To evaluate the effectiveness of intradermal injection of modified HA, a dynamic study of the morphological and functional state of the skin was carried out using non-invasive research methods, including pH-metry, sebometry, corneometry, elastometry, visoscanning, ultrasound scanning, and confocal laser scanning microscopy. The measurements were taken before the start of the treatment (initial skin condition) and 3-4 weeks after the completion of the procedures.
The study of morphological and functional parameters of the skin revealed age-related features. In the first group of patients aged 35 to 40 years, all the parameters studied before the procedure were within the normal range. In patients of the middle and older age groups, age-related deviations from normal values of varying severity were observed. Dehydration of the skin was especially pronounced. The average value of moisture in patients of the older age group (group 3) was 62% lower than in patients of group 1, and the difference in elasticity between groups 1 and 3 was 52%.
When using the preparation of HA modified with vitamin C and amino acids (glycine, proline, lysine), a significant increase in skin elasticity, a decrease in the severity of mimic and static wrinkles, restoration of the uniformity of complexion skin color, as well as leveling of the manifestations of xerosis (where present) was also noted in all patients.
The response to therapy was also different in different age groups. So, in patients aged 35-40 years, in whom all skin indicators were within the normal range before the start of the procedures, they approached the upper limit of the norm after the treatment. In the group from 41 to 55 years, therapy led to a more significant improvement in all studied parameters. In the group of patients aged 56-62 years (with predominance of signs of Glogau type III aging), the course of therapy led to the greatest increase in indicators, especially moisture and oiliness of the skin, compared with younger patients. Moisture increased by 87% and oil content by 46%. Moisture, fat content and pH of the skin characterize its barrier properties. Since it is known that women of the older age group have the most pronounced deficiency of antioxidants in blood and skin, the administration of HA modified with ascorbic acid led to a decrease in the intensity of lipid peroxidation in the skin, stabilization of cell membranes, normalization of skin pH, and as a result, its barrier properties, therefore leading to an increase in its hydration. However, according to an objective assessment of the morphofunctional state of tissues, after the therapy there was no compensation for existing age-related skin changes in this group of patients, since after the procedures, the indicators, despite the increase, still did not correspond to physiologically normal values. According to the subjective assessments by the patients themselves, 3 procedures were insufficient to compensate for the existing age-related skin changes.
A study of the skin microstructure after a course of procedures also showed a positive trend. According to the ultrasound scanning results, a more uniform structure of the epidermis was noted with a decrease in thinning areas, with more uniform fit to the dermal structures, which indicates the restoration of the morphofunctional state of this layer. From the side of the epidermis and dermis layer, there was an increase in thickness, more uniform distribution of structures, i.e. an increase in hyperechogenicity and a reduction in areas of hypoechogenicity, including thickening of the papillary zone of the dermis, which indicates the optimization of the functional state of the skin as a result of the action of the drug components.
When evaluating the relief of the skin through visoscanning, positive dynamics was noted for all the studied indicators. The best results after the course of therapy were obtained in group 1, where the patients initially had less pronounced changes compared to other groups. In group 2, there was also positive value dynamics so they approached the average microrelief parameters of group 1. In group 3, positive dynamics of all indicators was also noted, but the best results among all the studied indicators were obtained when testing the “smoothness” parameter. It increased by 81% in relation to the initial value (p less than 0.05) and approached the values of "smoothness" of the 1st group of patients, which was a consequence of the high moisturizing ability of the modified HA preparation.
Confocal laser scanning microscopy detected the main CLSM signs of involutional skin changes. At the level of the stratum corneum in all patients, along with characteristic non-nuclear polygonal cells, highly refractory white inclusions (scales) were determined, the number of which corresponded to the severity of skin xerosis, and a decrease in the height of the papillary dermis was noted. In older patients, the visualization of skin structures at the level of the upper layers of the dermis showed fixation of papillary capillaries in a smaller number than in younger subjects. On the deeper layers of the dermis, there were areas with intersecting filamentous structures of unequal thickness with varying degrees of architectural chaos, which reflects the severity of degradation of the intercellular framework in the dermis.
The general trend of CLSM skin pictures after 3 procedures of modified HA injections can be described as general improvement in the degree of layer refractoriness, i.e. improved optical properties of the tissue, which allows for exploring the deeper layers of the scan.
In group 1, according to the CLSM data, there was a complete leveling of the manifestations of xerosis (a decrease up to complete disappearance of white brightly colored polygonal structures on the surface of the stratum corneum – the scales). The architectonics of the skin's dermal layer did not undergo significant changes, which corresponds to the agerelated morphological features of aging- for this group.
In group 2, where the initial observations had shown more pronounced manifestations of skin dryness, a greater degree of refractoriness of structures at all scanning levels, the structure of the epidermis, the cellular structure of each layer according to the CLSM data after the course of therapy retained their morphological features without a tendency to any changes, which speaks about the safety of the technique.
In group 3, after the injection of modified HA, the phenomena of xerosis of the surface layers of the skin significantly decreased. this group showed the greatest manifestations of compensation for existing skin xerosis, the most pronounced symptom complex among all the observed patients. At the dermis level, there was an improvement in the qualitative characteristics of the mesh structure: the fibers had a more ordered arrangement with a tendency to a more uniform structure.
For an objective assessment of the data of CLSM - scanning, the parameters of the average thickness of the epidermis were studied, since they indirectly reflect the state of the hydroreserve and metabolic activity of the deeper layers of the skin. After the therapy, there was an improvement of the studied parameter in all 3 observation groups.
Thus, the conducted experimental and clinical studies have shown the safety and good tolerability of modified HA preparations. Stimulation of skin regeneration was shown when gels were administered to laboratory animals and good clinical efficacy for treating age-related skin changes in patients of different age groups. The proposed technique can be recommended for courses of anti-age therapy with correction of the duration and number of sessions according to the degree of involutional skin changes on a case-to-case basis.
FINDINGS
1. It was found that, upon subcutaneous administration, hydrogels of unmodified (native) HA underwent enzymatic lysis and macrophage resorption by day 3, while gels modified with vitamin C, amino acids, oligopeptides and folic acid did so by day 14 post-injection. No connective tissue capsule formed around the implant at any periods of the experiment, in contrast to the case of HA hydrogels modified with DE diols. Intradermal administration of modified HA hydrogels led to a pronounced proliferation of fibroblasts and neoangiogenesis, which, according to histochemistry and electron microscopy findings, was accompanied by an increase in the synthesis and fibrillogenesis of collagen and elastic fibers.
2. The results of clinical studies have shown a good clinical effect, safety and good tolerability of HA modified with vitamin C and a complex of amino acids. 100% of subjects with involutional changes in the skin and its appendages subjectively noted a significant positive effect: a decrease in the severity of mimic and static wrinkles, improvement in skin color, its tone and hydration. There were no adverse events or complications.
3. It was found that the use of modified HA resulted in the normalization of skin oil content and pH, plus a proven increase in skin elasticity and hydration. The maximum changes in indicators were observed in older patients, in whom skin oiliness increased by 46%, elasticity by 1.3 times, and moisture content by 87%.
4. The positive effect of the proposed technique on the structural components of the skin was proven and confirmed by the results of ultrasound scanning, visioscanning and confocal laser scanning microscopy. An increase in the thickness of the epidermis, epidermal junction and papillary dermis was noted, leading to an improvement in the skin microrelief in all age groups. In patients of the older age group, the most significant changes in the microrelief and microstructure of the skin were observed: the “smoothness” parameter of the skin increased by 81% compared to the initial value (p less than 0.05), elastin and collagen fibers had more ordered arrangement and a more uniform structure than before the course of procedures.
PRACTICAL RECOMMENDATIONS
The proposed technique can be recommended for mandatory inclusion in anti-age therapy programs for patients with involutive skin changes of varying severity. Patients of the older age group (aged 56-62 years, with predominance of signs of type III aging according to Glogau) and patients of the middle age group (41-55 years, type II aging according to Glogau) with signs of premature skin aging need individual adjustment of the course to increase the number of procedures, which will improve the morphological and functional parameters of the skin in these patients to reach the physiological norm.
BIBLIOGRAPHY
1. V.N. Anisimov, Molecular and physiological mechanisms of aging (Молекулярные и физиологические механизмы старения), In 2 vol. — 2nd ed. — St. Petersburg, Nauka, 2008. — Vol. 1. — Pg. 481. — Vol. 2. — Pg. 434.
2. A.N. Afanasyeva, Indicators of endogenous intoxication in elderly people//Clinical gerontology. (Показатели эндогенной интоксикации у людей пожилого возраста//Клиническая геронтология). – 2002. – Vol. 8. – No. 5. – Pg. 3-4.
3. V.V. Bazarny, I.E. Valamina, E.A. Tikhonina. Ribomunil immunomodulation of reparative processes in the skin of rats//Bulletin of Experimental Biology and Medicine (Иммуномодуляция рибомунилом репаративных процессов в коже крыс//Бюл. экспер. биол. и мед.) – 2007. – No. 143. – Pg. 660-662.
4. V.V. Burunova, N.E. Manturova, G.O. Smirnova, G.V. Stavitskaya, V.A. Stupin, K.N. Yarygin. Cellular technologies in facial skin revitalization//Breast Cancer (Клеточные технологии в ревитализации кожи лица//РМЖ) – 2009. – No. 17. – Pg. 1058-1061.
5. M. Vaki, K. Miyamoto. Photocured gel based on cross-linked hyaluronic acid and method for its preparation. (Фотоотвержденный гель на основе сшитой гиалуроновой кислоты и способ его получения). Russian Federation Patent 2003, No. 2197501.
6. T.Yu. Vitruk. Peculiarities of change in cell-matrix relationships in the skin during chronological and photoinduced aging: (Особенности изменений клеточно-матриксных взаимоотношений в коже при ее хронологическом и фотоиндуцированном старении) Abstract of dissertation for Cand. Med. degree: 14.00.16., 14.00.11. – Tomsk, 2008. – 25 pg.
7. V.L. Voyenkov. Reactive oxygen species: pathogens or healers?//Clinical gerontology. (Активные формы кислорода – патогены или целители?//Клиническая геронтология) – 2003. – No. 3. – Pg. 27-40.
8. E.A. Voyton. Correction of age-related skin changes with peptide bioregulators: (Коррекция возрастных изменений кожи пептидными биорегуляторами) Abstract of dissertation for Cand. Med.: 14.00.53. – St. Petersburg, 2005. – 21 pg.
9. I.V. Gavrilov. The impact of various gas regimes on the state of lipid peroxidation under conditions of age-related involution: (Воздействие различных газовых режимов на состояние перекисного окисления липидов в условиях возрастной инволюции) Abstract of dissertation for Cand. Biol. degree 14.00.16. – Ekaterinburg, 2000. – 24 pg.
10. V. Garcia, C. Beltran, F. Bouffard, G. Rokka. Mesotherapy of aging skin of men and women//Injection methods in cosmetology. (Мезотерапия стареющей кожи мужчин и женщин//Инъекционные методы в косметологии) – 2009. – October. – Pg. 14-20.
11. G.P. Gladyshev. Supramolecular thermodynamics of genes and aging//Advances in gerontology. (Супрамолекулярная термодинамика генов и старение//Успехи геронтологии.) – 1999. – No. 3 – Pg. 30-31.
12. E.I. Gubanova. Involutional changes in the skin of the lower third of the face (clinical and functional study) (Инволюционные изменения кожи нижней трети лица (клинико-функциональное исследование)) Abstract of dissertation for Doc. Med.: 14.01.10. – Moscow, 2010. – 44 pg.
13. A.G. Gunin, N.K. Kornilova, V.V. Petrov, O.V. Vasilieva. Age-related changes in the number and proliferation of fibroblasts in human skin//Advances in Gerontology. (Возрастные изменения численности и пролиферации фибробластов в коже человека//Успехи геронтологии.) – 2011. – Vol. 24. - No. 1, – Pg. 43-47.
14. V.A. Gusev. Free radical theory of aging in the paradigm of gerontology//Advances in gerontology. (Свободнорадикальная теория старения в парадигме геронтологии//Успехи геронтологии.) – 2000. – No. 4. – Pg. 22-26.
15. A.I. Deev, E. Chaikovskaya. Factors of accelerated skin aging//Injection methods in cosmetology. (Факторы ускоренного старения кожи//Инъекционные методы в косметологии.) – 2009. – Oct. – Pg. 4-12.
16. V.I. Dontsov. Fundamental mechanisms of aging: opportunities for assessing the "true age" and influences on it//Yearbook of the National Gerontological Center. (Фундаментальные механизмы старения: возможности для оценки «истинного возраста» и влияний на него//Ежегодник национального геронтологического центра.) – 2000. – No. 3. – Pg. 22-29.
17. V.I. Dontsov, V.N. Krutko, A.A. Podkolzin. Fundamental mechanisms of geroprophylaxis. (Фундаментальные механизмы геропрофилактики.) – M.: Bioinformservis, 2002. – 464 pg.
18. V.A. Dubinskaya, E.V. Vinogradova. Human skin: moisture exchange and aging//Clinical gerontology. (Кожа человека: влагообмен и старение//Клиническая геронтология.) – 2000. – No. 7-8. – Pg. 22-26.
19. E.Yu. Ermakova. Lipid peroxidation and the barrier function of the skin in aging conditions: (Перекисное окисление липидов и барьерная функция кожи в условиях старения организма) Abstract of dissertation for Cand. Med.: 03.00.04, 14.00.16. – Ekaterinburg, 2005. – 23 pg.
20. E.A. Efimov. Characteristics of the completeness of skin regeneration// Morphology. (Характеристика полноты регенерации кожи//Морфология.) – 2000. – No. 3. – Pg. 45.
21. G.P. Zhizhina. The role of apoptosis in normal ontogenesis, pathogenesis and aging//Clinical gerontology. (Роль апоптоза в нормальном онтогнезе, патогенезе и старении//Клиническая геронтология.) – 2002. – No. 4 – Pg. 3-10.
22. N.S. Zhirnova. Morphofunctional study of biogenic amines of skin structures after the introduction of hyaluronic acid preparations: (Морфофункциональное исследование биогенных аминов структур кожи после введения препаратов гиалуроновой кислоты) Abstract of dissertation for Cand. Biol. degree 03.00.25. – Cheboksary, 2007. – 23 pg.
23. V.V. Zapadnyuk, L.P. Kuprash, M.U. Zaika. The effectiveness of geriatric drugs containing natural metabolites in studies on animals of different ages // Bulletin of the USSR Academy of Medical Sciences.(Эффективность гериатрических препаратов, содержащих естественные метаболиты, в исследованиях на животных разного возраста//Вестник АМН СССР) – 1990. – No. 1. – Pg. 28-31.
24. A.N. Zimnitsky. Glycosaminoglycans in the biochemical mechanisms of aging: (Гликозаминогликаны в биохимических механизмах старения) Abstract of dissertation for Doct. Biol.: 03.00.04. – Tyumen, 2005. – 51 pg.
25. E.V. Ivanova. Pathogenetic substantiation of the use of oxygen-ozone mixture in the correction of age-related skin changes: (Патогенетическое обоснование применения кислородно-озоновой смеси в коррекции возрастных изменений кожи) Abstract of dissertation for Cand. Biol. degree 14.00.11. – Moscow, 2007. – 26 pg.
26. L.D. Kalyuzhnaya, S.I. Sharmazan, E.V. Moiseeva, I.N. Bondarenko. The place of hyaluronic acid in the correction of involutional skin changes//Aesthetic Medicine. (Место гиалуроновой кислоты в коррекции инволюционных изменений кожи//Aesthetic Medicine.) – 2009. – No. 4. – Pg. 44-46.
27. E. Karpova, S. Ananan. Peculiarities and criteria for selecting patients for injectable contouring with the use of various implant materials//Abstracts (Особенности и критерии отбора пациентов для контурной инъекционной пластики с ипользованием различных имплантационных материалов//Тез.докл.) 5th International Congress on Aesthetic Medicine. – Moscow, 2005. – Pg. 97-98.
28. I.I. Kolgunenko. Fundamentals of gerontocosmetology. (Основы геронтокосметологии.) – M.: Meditsina, 1974. – 224 pg.
29. V.K. Koltover. Free radical theory of aging: historical essay//Advances in gerontology. (Свободнорадикальная теория старения: исторический очерк//Успехи геронтологии.) – 2000. – No. 4. – Pg. 273-282.
30. A.N. Komarnitsky. Prevention of aging: protocols for the prevention of premature aging: current state and prospects // Yearbook of the National Gerontological Center. (Профилактика старения: протоколы предупреждения преждевременного старения: современное состояние и перспективы//Ежегодник национального геронтологического центра.) – 2000. – No. 3. – С.79–84.
31. Yu.V. Konev, L.B. Lazebnik. Metabolism of endotoxin in the body and its role in the process of involution//Clinical gerontology. (Метаболизм эндотоксина в организме и его роль в процессе инволюции//Клиническая геронтология.) – 2009. – Vol. 15. – No. 4 – Pg. 39-46.
32. G.G. Konovalov, V.Z. Lankin, A.K. Tikhaze, I.B. Nezhdanova, M.O. Lisina, A.V. Kukharchuk. Complex of antioxidant vitamins protects blood plasma LDL from free radical oxidation and increases the activity of antioxidant enzymes in the erythrocytes of ischemic disease patients//Bulletin of Experimental Biology and Medicine (Комплекс витаминов-антиоксидантов защищает ЛПНП плазмы крови от свободнорадикального окисления и повышает активность антиоксидантных ферментов в эритроцитах больных ИБ//Бюл.экспер.биол. и мед.) – 2003. – No. 8. – Pg. 165-166.
33. R.V. Korgunova. The study of the mechanism of physiological aging of facial skin based on its morphological and TAPE-STRIPPED methods // Bulletin of dermatology and venereology. (Изучение механизма физиологического старения кожи лица на основании ее морфологического и TAPE-STRIPPED методов//Вестник дерматологии и венерологии.) – 2005. – No. 6. – Pg. 65-70.
34. G.P. Kotelnikov, O.G. Yakovlev, N.O. Zakharova. Gerontology and geriatrics. (Геронтология и гериатрия.) – M., Samara:Samar. Dom Pechati, 1997. – 798 pg.
35. N.G. Lapatina. Modern trends in biorevitalization//Bulletin of Aesthetic Medicine (Современные тенденции биоревитализации // Вест.эстетич.мед.) – 2008. – No. 1. – Pg. 34-39.
36. A.V. Mayorova, A.I. Deev, G.A. Timofeev. Changes in skin microrelief in smoking and non-smoking women of different ages//Advances in gerontology. (Изменения микрорельефа кожи у курящих и некурящих женщин разного возраста//Успехи геронтологии.) – 2008. – No. 2. – Pg. 226-230.
37. O.G. Makeev. The use of autologous dermal fibroblasts enriched in multipotent mesenchymal stromal cells for the treatment of neurotrophic leg ulcers in patients with type ll diabetes // Bulletin of the Ural Medical Academic Science. (Применение аутологичных дермальных фибробластов, обога- щенных мультипотентными мезенхимальными стромальными клетками, для лечения нейротрофических язв конечностей у больных сахарным диабетом ll типа//Вестник Уральской медицинской академической науки) – 2010. – No. 1. – Pg. 49-52.
38. A.V. Makrushin. Reverse development and senile involution.//Advances in gerontology. (Обратное развитие и старческая инволюция.//Успехи геронтологии.) – 2003. – No. 11 – Pg. 47-48.
39. A.A. Margolina, E.I. Hernandez, O.E. Zaikina. New cosmetology. (Новая косметология.) – М.:Kosmetika i Meditsina, 2001. – 204 pg.
40. S.V. Moskvin, E.V. Antipov, E.G. Zarubina, E.A. Ryazanova. Low-intensity laser radiation and hyaluronic acid laser phoresis as methods for correcting age-related skin changes: further expansion of the evidence base//Cosmetics & Medicine. (Низкоинтенсивное лазерное излучение и лазерофорез гиалуроновой кислоты как методы коррекции возрастных изменений кожи: дальнейшее расширение доказательной базы//Косметика & медицина.) – 2011. – No. 2. – Pg. 34-40.
41. Yu.A. Ovchinnikov. Bioorganic chemistry. (Биоорганическая химия.) – M.:Prosveschenie, 1987. – 815 pg.
42. O.S. Ozerskaya. Mesotherapy in dermatocosmetology. (Мезотерапия в дерматокосметологии.) – St. Petersburg, Iskusstvo Rossii, 2009. – 224 pg.
43. A.M. Olovnikov. Intranuclear ion fountains as regulators of genome activity: the dominant fountain hypothesis and some epigenetic effects//Molecular biology. (Внутриядерные ионные фонтаны как регуляторы деятельности генома: фонтанная гипотеза доминанты и некоторые эпигенетические эффекты//Молекулярная биология.) – 2000. – Vol. 35. – Pg. 163-176.
44. A.M. Olovnikov. Redusome theory of aging and control of biological time in individual development//Biochemistry. (Редусомная теория старения и контроля биологического времени в индивидуальном развитии//Биохимия.) – 2003. – Vol. 68 – No. 1. – Pg. 7-41.
45. E.N. Parsagashvili. Geroprotective technologies in aesthetic medicine//Cosmetics and medicine. (Геропротективные технологии в эстетической медицине//Косметика и медицина.) – 2006. – No. 1. – Pg. 70-76.
46. Yu.N. Perlamutrov, K.B. Olkhovskaya. Mechanisms for the development of skin changes in old age // Yearbook of the National Gerontological Center. (Механизмы развития изменений в коже в пожилом возрасте//Ежегодник национального геронтологического центра.) – 2001. – No. 4. – Pg. 49-52.
47. A.A. Podkolzin, V.I. Dontsov. et al. Antioxidant protection of the body during aging and some pathological conditions associated with it//Clinical gerontology. (Антиоксидантная защита организма при старении и некоторых патологических состояниях, с ним связанных//Клиническая геронтология.) – 2001. – Vol. 7. – No. 3-4. – Pg. 50-58.
48. Elderly person in the modern world. (Пожилой человек в современном мире.) An almanach. Edited by Yu.A. Scherbuk, A.N. Rzhanenkov, V.N. Anisimov, V.H. Khavinson – St. Petersburg, OOO IPK KOSTA, 2008. — 256 pg.
49. V.A. Polyakova, I.M. Kvetnoy. Thymus and aging. Neuroimmunoendocrine mechanisms. (Тимус и старение. Нейроиммуноэндокринные механизмы.) – St. Petersburg.: Sistema, 2004. – 102 pg.
50. A.I. Potapenko, A.P, Akifyev. On the way to the search for the program and the initial substrate of aging//Advances in gerontology. (На пути поиска программы и инициального субстрата старения//Успехи геронтологии.) – 1999. – No. 3. – Pg. 24-32.
51. N.N. Potekayev, D.V. Gutkin. New approaches to the correction of age-related skin changes//Clinical dermatology and venereology. (Новые подходы к коррекции возрастных изменений кожи//Клиническая дерматология и венерология.) – 2003. – No. 1. – Pg. 18-21.
52. I.F. Radaeva, G.A. Kostina. Hyaluronic acid: biological role, structure, synthesis, isolation, purification and application (review)//Applied Biochemistry and Microbiology. (Гиалуроновая кислота: биологическая роль, структура, синтез, выделение, очищение и применение (обзор)//Прикл. биохим. микро-биол.) – 1997. – Vol. 33. – No. 2. – Pg. 133-137.
53. E.A. Ranneva, E. Chaikovskaya. Applied possibilities of antihomotoxic therapy (Прикладные возможности антигомотоксической терапии) Les Nouv. Esthet. – 2006. – No. 11. – Pg. 60-63.
54. V.G. Rebrov, O.A. Gromova. Vitamins, macro- and microelements. (Витамины, макро- и микроэлементы.) – М.: GEOTAR-Media, 2008. – 960 pg.
55. A.R. Rozhanets. Comprehensive correction of age-related changes in the face using myostimulation and mesotherapy: (Комплексная коррекция возрастных изменений лица с использованием миостимуляции и мезотерапии) Abstract of dissertation for Cand. Med.: – Moscow, 2010. – 22 pg.
56. I.S. Rolik. Fetal organ preparations: clinical application. (Фетальные органопрепараты: клиническое применение.) – М.: RegBioMed, 2003. – 763 pg.
57. T.G. Ruksha, V.V. Salamatin, I.A. Savchenko. Intradermal injection of ascorbic acid to correct changes caused by ultraviolet radiation (Внутрикожное введение аскорбиновой кислоты для коррекции изменений, вызванных ульрафиолетового излучения) Mesotherapy. – 2008. – No. 4. – Pg. 8-12.
58. G.A. Ryzhak, S.S. Konovalov. Geroprotectors in the prevention of age-related pathology. (Геропротекторы в профилактике возрастной патологии.) – SPb: Prime-Euroznak, 2004. – 160 pg.
59. E.S. Severin, T.L. Aleinikova, E.V. Osipov, S.A. Silaeva. Biological Chemistry. (Биологическая химия.) – M.: Meditsinskoe Informatsionnoe Agentstvo, 2008. – 364 pg.
60. V.I. Semkin. Sunlight as a factor in skin aging. (Солнечный свет как фактор старения кожного покрова.) Elderly Patient. Life Quality. («Пожилой больной. Качество жизни.»// Abstracts. 7th International Science and Practice Conference. – Moscow, 2002. – Pg. 85.
61. V.P. Skulachev. Aging as an atavistic program that can be tried to cancel // Bulletin of the Russian Academy of Sciences. (Старение как атавистическая программа, которую можно пытаться отменить//Вестник РАН.) – 2005. – Vol. 75. – Pg. 831-843.
62. I.O. Smirnova and I.M. Kvetnoi. Photoaging of the skin – molecular markers in the epidermis and dermis // Advances in Gerontology. (Фотостарение кожи – молекулярные маркеры эпидермиса и дермы//Успехи геронтологии.) – 2004. – No. 13. – Pg. 44-51.
63. O.V. Smirnova. Neuroimmunoendocrine markers of skin aging: (Нейроиммуноэндокринные маркеры старения кожи) Abstract of dissertation for Doc. Biol. degree 14.00.53. – St. Petersburg, 2004. – 46 pg.
64. So San Ho, G.A. Rebrova, V.K. Vasilevsky, Yu.A. Romanov, V.A. Bykov, L.B. Rebrov. Collagen changes during aging in vitro//Clinical Gerontology. (Изменения коллагена при старении in vitro//Клиническая геронтология). – 2001. – No. 3-4. – Pg. 35-40.
65. I.V. Tikhonova. Study of the regulation of blood flow in the microvasculature of human skin during aging: (Исследование регуляции кровотока в микроциркуляторном русле кожи человека в процессе старения) Abstract of dissertation for Cand.Biol. degree: 03.00.13. – Puschino, 2006. – 21 pg.
66. D.M. Faller, D. Shields. Molecular biology of the cell. (Молекулярная биология клетки.) – М.:Binom-Press, 2004. – 256 pg.
67. I.A. Fedorischev. Biophysical characteristics of modified hyaluronic acid: (Биофизическая характеристика модифицированной гиалуроновой кислоты) Abstract of dissertation for Cand.Biol. degree: 03.00.02, 05.13.01. – Tula, 2006. – 18 pg.
68. G.I. Fisenko, O.M. Burylina, L.V. Parkaeva, V.K. Ermolaev et al. Aesthetic imperfections of the skin (Эстетические недостатки кожи). Ed. by V.A. Vissarionov. – М.:Binom, 2006. – Pg. 18-23.
69. D.E. Fitzpatrick, D.A. Elling. Secrets of dermatology (Секреты дерматологии.) Translated from English. – SPb.: Nevsky Dialekt, 1999. – 511 pg.
70. V.V. Frolkis. Aging and increasing life expectancy. (Старение и увеличение продолжительности жизни.) – L.: Nauka, 1988. – 241 pg.
71. V.N. Khabarov, A.N. Zelenetsky. Nanotechnological reticulation of hyaluronic acid. (Нанотехнологическая ретикуляция гиалуроновой кислоты.//КИ.) – 2008. – Vol. 2. – Pg. 8-14.
72. V.N. Khabarov, P.Ya. Boikov, M.A. Selyanin. Hyaluronic acid. (Гиалуроновая кислота.) – M.: Prakticheskaya Meditsina, 2012. – 224 pg.
73. V.H. Havinson, A.G. Golubev. Aging of the epiphysis//Advances in gerontology. (Старение эпифиза//Успехи геронтологии.) – 2002. – No. 9. – Pg. 67-72.
74. K.A. Khadartseva, A.A. Khadartsev, E.A. Ryazanova, N.S. Rudneva. New methods of diagnostics and restorative treatment//Fundamental research. (Новые способы диагностики и восстановительного лечения//Фундаментальные исследования.) – 2007. – No. 11. – Pg. 127-128.
75. K.P. Hanson. The role of apoptosis in aging and age-related pathology//Advances in Gerontology. (Роль апоптоза в старении и возрастной патологии//Успехи геронтологии.) – 1999. – No. 3. – Pg. 103-110.
76. A.M. Chernukh. The skin and its importance in the life of the organism (Instead of an Introduction) (Кожа и ее значение в жизнедеятельности организма (вместо введения))//Skin (Kozha). Ed. by A.M. Chernukh, E.P. Frolov. - M.: Meditsina, 1982. - Pg. 7 – 19.
77. E.V. Shvetsova. Pathophysiological mechanisms of skin regeneration under the action of mesenchymal cells (Патофизиологические механизмы регенераци кожи под действием мезенхимальных клеток) Abstract of dissertation for Cand. Med.: 14.00.16. 03.00.25. – Moscow, 2009. – 26 pg.
78. E.O. Sheveleva, I.A. Komarova, E.A. Orlova, M.V. Samoilov. Apoptosis and some features of inflammatory reaction on the skin during mesotherapeutic administration of drugs. (Апоптоз и некоторые особенности воспалительной реакции на коже при мезотерапевтическом введении препаратов) Bulletin of RUDN. – 2006. – No. 1. – Pg. 6-11.
79. M.A. Shirshakova. Clinical efficacy of skin stimulation with native hyaluronic acid preparations in the mirror of instrumental diagnostics//Injection methods in cosmetology. (Клиническая эффективность стимуляции кожи препаратами нативной гиалуроновой кислоты в зеркале инструментальной диагностики//Инъекционные методы в косметологии.) – 2010. – No. 3 – Pg. 74-81.
80. I.V. Shishkova. Immunomodulatory effect of heteropolysaccharides of various species origin in local temperature damage to the skin (Иммуномодулирующее действие гетерополисахаридов различного видового происхождения при локальных температурных повреждениях кожных покровов) Abstract of dissertation for Cand. Med.: 14.00.36. – Kursk, 2000. – 23 pg.
81. E.A. Shuginina. Injectable implants – pros and cons. (Инъекционные импланты – за и против)//LNE. – 2002 – No. 1. – Pg. 68-70.
82. E.I. Hernandez, E.I. Gubanova. E.Z. Parasagashvili. Injection methods in aesthetic medicine. (Инъекционные методы в эстетической медицине.) – М.:Kosmetika i Meditsina, 2010. – 199 pg.
83. K.N. Yarygin, V.V. Burunova, V.A. Stupin, G.O. Smirnova, N.E. Manturova, G.V. Stavitskaya. Cellular technologies in facial skin revitalization//Breast Cancer (Клеточные технологии в ревитализации кожи лица//РМЖ) – 2009. – No. 17. – Pg. 1058-1062.
84. Adams J.M., Cory S. et al. The Bcl-2 protein family: arbiters of cell survival. Science. – 1998. - Aug 28;281(5381):1322-6.
85. Alessandrini A, Di Bartolo C, Pavesio A, Pressato D. ACP gel: a new hyaluronic acid-based injectable for facial rejuvenation. Preclinical data in a rabbit model. Plast Reconstr Surg. – 2006. - Aug;118(2):341-6.
86. Amin SP, Phelps RG, Goldberg DJ. Mesotherapy for facial skin rejuvenation: a clinical, histologic, and electron microscopic evaluation. Dermatol Surg. - 2006 Dec;32(12):1467-72.
87. Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD. Photoaging: prevention and topical treatments. Am J Clin Dermatol. – 2010. – 11(2):95-102.
88. Aruffo A., Stamenkovic I., Melnick M. et al. CD44 is the principal cell surface receptor for hyaluronate. Cell. - 1990.- 61:1303—1313.
89. Atiyeh BS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesthetic Plast Surg. – 2008.- Nov;32(6):842-9.
90. Balin AK, Pratt L. Oxygen modulates the growth of skin fibroblasts. In Vitro Cell Dev. Biol. Anim. - 2002. - 38:305-10.
91. Baspevras M., Rouvrais C., Liégard L. et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res 2013; 29th May. [Electronic publication]
92. Benedetto AV. The environment and skin aging. Clin Dermatol. – 1998. - Jan- Feb;16(1):129-39.
93. Berneburg M. Research in practice: More than skin deep: aging of subcutaneous fat tissue. Dtsch Dermatol Ges. – 2010. - Oct;8(10):776-8.
94. Bland J. H., Cooper S. M. Osteoarthritis: a review of the cell biology involved and evidence for reversibility. Management rationally related to known genesis and pathophysiology. Semin Arthritis Rheum. - 1984. - 14:106—133.
95. Bowen AR, Hanks AN, Allen SM, Alexander A, Diedrich MJ, Grossman D. Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol. – 2003. - Jan;120(1):48-55.
96. Brawan J. Short- and long-term histologic effects of topical tretinoin on photodamaged skin. Int J Dermatol. – 1998.- Apr;37(4):286-92.
97. Calleja-Agius J, Brincat M. The effect of menopause on the skin and other connective tissues. - 2012 Apr;28(4):273-7.
98. Carruthers J, Cohen SR, Joseph JH, Narins RS, Rubin M. The science and art of dermal fillers for soft-tissue augmentation. . J Drugs Dermatol. - 2009. - Apr;8(4):335-50.
99. Chan E.L., Murphy J.T. Reactive Oxygen Species Mediate Endotoxin-Induced Human Dermal Microvascular Endothelial NF-kB Activation .Journal of Surgical Research. - 2003. - №1:120-126.
100. Chung JH, Hanft VN, Kang S. Aging and photoaging. J Am Acad Dermatol. – 2003. - Oct;49(4):690-7.
101. Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. – 2002. - Nov;22(6):555-7.
102. Cosgrove M.C., Franco O.H., Granger S.P., Murray P.G., Mayes A.E. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. - 2007. - Oct;86(4):1225-31.
103. Crimmins E.M., Finch C.E. The genetics of age-related health outcomes. J Ger- ontol A Biol Sci Med Sci. - 2012 May;67(5):467-9.
104. Dalle C. M., Pathak M.A. Skin photosensitizing agents and the role of reactive oxygen species in photoaging. J. Photochem. Photobiol. 2002;14(1-2):105-124.
105. Damiano R, Quarto G, Bava I, Ucciero G, De Domenico R, Palumbo MI, Autorino R. Prevention of Recurrent Urinary Tract Infections by Intravesical Administration of Hyaluronic Acid and Chondroitin Sulphate: A Placebo-Controlled Randomised Trial. Eur Urol. – 2011. - Jan (18);201-9.
106. Danby FW. Nutrition and aging skin: sugar and glycation. Сlin Dermatol. – 2010. - Jul-Aug;28(4):409-11.
107. Della Valle F., Romeo A. Hyaluronic acid fractions having pharmaceutical activity, and pharmaceutical compositions containing the same. Patent US. – No. 5925626. - 1999.
108. Di Pietro., Di Sante G. II recupero dlell elasticita e del turgore cutaneo mediante iniezione intradermica di acido hialuronico lal system Giornal Italiano di Dermatologia e Venerologia.- 2001. – 6.; 187-191.
109. Duranti F., Salti G., Bovani B. et al. Injectable hyaluronic acid gel for soft tissue augmentation. The clinical study. Dermatol. Surg. – 1998 - 24 (12): 1317-1325.
110. Egren U. M, Tammi R. H, Tammi M. I. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med 1997; 23:996–1001.
111. Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. – 2008. - May;144(5):666-72.
112. Fitzpatrick R.E. Endogenous growth factors as cosmeceuticals. Dermatol Surg. – 2005. - Jul;31(7 Pt 2):827-31.
113. Flynn TC, Sarazin D, Bezzola A, Terrani C, Micheels P. Comparative Histology of Intradermal Implantation of Mono and Biphasic Hyaluronic Acid Fillers. Dermatol Surg. – 2011. - May;37(5):637-43.
114. Frost, S. Weigel, P. Binding of hyaluronic acid to mammalian fibrinogens. Bio- chim. Biophys. Acta.- 1990.-V.1034.:39-45.
115. Fujii N, Kaji Y, Fujii N, Nakamura T, Motoie R, Mori Y, Kinouchi T. Collapse of homochirality of amino acids in proteins from various tissues during aging. Chem Biodivers. - 2010. - Jun;7(6):1389-97.
116. Gensanne D, Josse G, Schmitt AM, Lagarde JM, Vincensini D. In vivo visualization of hyaluronic acid injection by high spatial resolution T2 parametric magnetic resonance images. Skin Res Technol. – 2007/- Nov;13(4):385-9.
117. Gillardon F, Moll I, Meyer M, Michaelidis TM. Alterations in cell death and cell cycle progression in the UV-irradiated epidermis of bcl-2-deficient mice. Cell Death Differ. – 2003. - Jan;6(1):55-60.
118. Giunta S. Exploring the complex relations between inflammation and aging (in-flamm-aging): anti-inflamm-aging remodelling of inflamm-aging, from robustness to frailty. Inflamm Res. – 2008. - Dec;57(12):558-63.
119. Glogau R.G. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. – 1996. - Sep;15(3):134-8.
120. Grewe M. Chronological ageing and photoageing of dendritic cells. Clin Exp Dermatol. – 2001. - Oct;26(7):608-12.
121. Guinot C., Malvy D.J., Ambroisine L., Latreille J., Mauger E., Tenenhaus M., Morizot F., Lopez S., Le Fur I., Tschachler E. Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score.Arch Der- matol. – 2002. - Nov;138(11):1454-60.
122. Harman D. Free-radical theory of aging: invreasing the functional life span. Ann. N. Y. Acad.Sci. 1994; 717: 1— 15.
123. Harrison K. Introduction to polymeric drug delivery systems. In Biomedical polymers / ed. by M. Jenkins. -Publishing England, Limited Cambridge: CRC Press. - 2007. -236 p.
124. Hayflick L. DNA replication and traintracks. Science. 2002; May 31; 296(5573):1611-2.
125. http://www.plasticsurgery.org
126. Jackson D. G. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. - 2004.- 112:526—538.
127. Karen L. Goa, Paul Benfield: Hyaluronic acid. A Review of its Pharmacology and Use as a Surgical Aid in Ophtalmology and its Therapeutic Potential in Joint Disease and Wound Healing. Drugs. 1994. - 47 (3): 536— 566.
128. Kerscher M., Bayrhammer J., Reuther T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol Surg. - 2008. May;34(5):720-6.
129. Krutmann J., Morita A., Chung JH. Sun exposure: what molecular photodermatology tells us about its good and bad sides. Invest Dermatol. – 2012. - Mar;132(3 Pt 2):976-84.
130. Kwon O.S., Mesotherapy for Facial Rejuvenation. In Shiffman M.A., Mirrafati S. J., Lam S.M., Cueteax C.G.Simplified Facial Rejuvenation. Berlin Heidelberg, N- Y: Springer. 2008, p. 26-41.
131. Lacarrubba F., Nedeschi A.. Nardone B., Micali G. Mesotherapy for skin rejuvenation assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-section B-mode scanning. Dermatologic Therapy. - 2008. -Vol 21 №3. – Р.1–5.
132. Lee J.H.An HT Chung JH et al Acute effects oF UVB radiation on the proliferation and differentiation of keratinocytes.Photodermatol Photoimmunol Photomed/- Vol. 18. - №5. – Р.253–261.
133. Levenberg A., Halachmi S., Arad-Cohen A., Ad-El D., Cassuto D., Lapidoth M. Clinical results of skin remodeling using a novel pneumatic technology. Int . J. Dermatol. – 2010. - Dec;49(12):1432-9.
134. Linker A. Meyer K Production of unsaturated urodnides by bacterial hyaluronidases Nature. - 1954. - Vol174. – р.1192–1194.
135. López-Torres M., Barja G., Calorie restriction, oxidative stress and longevity .Esp Geriatr Gerontol. – 2008. - Jul-Aug;43(4):252-60.
136. Lowe NJ, Grover R. Injectable hyaluronic acid implant for malar and mental enhancement. Dermatol Surg. – 2006. - Jul;32(7):881-5.
137. Lussow A.R.,Buelow R. Use of HA as an immunosuppressant. Patent №601364145 US 1999.
138. Ma W, Wlaschek M, Tantcheva-Poór I, Schneider LA, Naderi L, Razi-Wolf Z, Schüller J, Scharffetter-Kochanek K.Ma W. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue.Clin Exp Dermatol. – 2001. - Oct;26(7):592-9.
139. Masaki H, Okano Y, Sakurai H. Generation of active oxygen species from advanced glycation end-products (AGE) under ultraviolet light A (UVA) irradiation. Biochem. Biophys. Res. Commun. 1997; Jun 18;235(2):306-10.
140. Masaki H. Role of antioxidants in the skin: anti-aging effects.J. Dermatol Sci. - 2010. - May;58(2):85-90.
141. Millis A. Differetial expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblaste. Exp. Cell. Res. -1992. - 201:373-378.
142. Moffat F. L. Jr., Han T., Li Z. M. et al. Involvement of CD44 and the cytoskeletal linker protein ankyrin in human neutrophil bacterial phagocytosis. J Cell Physiol. - 1996. - 168:638—647.
143. Naru E., Ohta T., Inomata K. et al. Donor age-dependent acceleration of cellular aging by repeated ultraviolet A irradiation of human dermal fibroblasts derived from a single donor .Hum. Cell - 2009. - Vol. 22. P. 31–37.
144. Odetti PR, Borgoglio A, Rolandi R. Age-related increase of collagen fluorescence in human subcutaneous tissue. Metabolism. – 1992. - Jun;41(6):655-8.
145. Oxlund N., Anderssen T. The roles of hyaluronic acid collagrn and elastin in thr mechanical properties of connective tissue. J.anat. – 1980 – 131:611-620.
146. Passi S, De Pità O, Grandinetti M, Simotti C, Littarru GP Passi S. The combined use of oral and topical lipophilic antioxidants increases their levels both in sebum and stratum corneum.Biofactors. - 2003. - 18(1-4):289-97.
147. Paul H. Weigel, P. The spacific interaction between fibrin(ogen) and hyaluronan: possible consenquences in haemostases, inflammation and wound healing. The biology of hyaluronan. Wiley, Chichester . – 1989. - p.248—264.
148. Peat R. Oral absorption of progesterone. J Natl Med Assoc. – 1986. - Mar;78(3):182, 185.
149. Pierre A, Levy PM. Hyaluronidase offers an efficacious treatment for inaesthetic hyaluronic acid overcorrection. J Cosmet Dermatol. – 2007. - Sep;6(3):159-62.
150. Reiter RJ, Tan DX. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. – 2003. -Cardiovasc Res. – 58(1):10-19.
151. Rhie G, Shin M.H., Seo J.Y., et al. - Aging- and photoaging-dependent changes of enzymic and nonenzymic antioxidants in the epidermis and dermis of human skin in vivo. J. Invest. Dermatol. – 2001 - 117 (5): 1212–1217.
152. Rittié L., Fisher G.J. UV-light-induced signal cascades and skin aging. Ageing Res Rev. – 2002. - Sep;1(4):705-20.
153. Schandz W, Schippet W .,Ulmer A Arterial embolization caused by injection of hyaluronic acid (Restylane). British J.of Derm. - 2002. – 146:928-929.
154. Scott J.E., Presti D. Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl radicals is dependent on hyaluronan molecular mass. Cell Biochem Funct. – 1994. - Dec;12(4):281-288.
155. Sell D.R., Nemet I., Monnier VM. Partial characterization of the molecular nature of collagen-linked fluorescence: role of diabetes and end-stage renal disease.Arch Biochem Biophys. - 2010. - Jan 15;493(2):192-206.
156. Shindo Y., Akiyama Y., Yamazaki Y.,Satio K., Takase Y., Exp.Gerontol . – 1991. - №1:29-35
157. Sunderkötter C, Kalden H, Luger TA. Aging and the skin immune system. Arch. Dermatol. 1997; Oct;133(10):1256-62.
158. Tesar B. M., Jiang D., Liang J. et al. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. - 2006. - 6:2622—2635.
159. Thorne CH. Discussion. Do plastic surgeons have cosmetic surgery? Plast Reconstr Surg. – 2009. - Dec;124(6):2170.
160. Tomihata K, Ikada Y. Crosslinking of hyaluronic acid with water-soluble carbodiimide. J Biomed Mater Res. - 1997. - Nov;37(2):243-51.
161. Toole B. P. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. - 2004. - 4:528—539.
162. Tsai T.C., Hantash BM Cosmeceutical Agents:A Comprehensive Review of the Literature. Clinical. Medicine: dermatology. – 2008. – No. 1. – 1-20.
163. Urschitz J, Iobst S, Urban Z, Granda C, Souza KA, Lupp C, Schilling K, Scott I, Csiszar K, Boyd CD. A serial analysis of gene expression in sun-damaged human skin. J Invest Dermatol. - 2002. - Jul;119(1):3-13.
164. Verschooten L, Claerhout S, Van Laethem A, Agostinis P, Garmyn M. New strategies of photoprotection. Photochem Photobiol. – 2006. - Jul-Aug;82(4):1016- 23.
165. Verzár F. Basic research in experimental gerontology. Experientia. - 1976- Jun 15;32(6):746-7.
166. Walsh N. M., Prokopetz R., Tron V. A. et al. Histopathology in erythroderma: review of a series of cases by multiple observers. J Cutan Pathol. - 1994. - 21:419— 423.
167. Wang F. Garza L.A. et al. In vivo stimulation of de novo collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J. Invest. Dermatol. – 2004. - 122:1471-1479.
168. Wang F., Garza L.A., Kang S., Varani J., Orringer J.S., Fisher GJ, Voorhees J.J. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. – 2007. - Feb;143(2):155-63.
169. Weindl G, Schaller M, Schäfer-Korting M, Korting HC. Hyaluronic acid in the treatment and prevention of skin diseases: molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol Physiol. – 2004. - Sep Oct;17(5):207-13.
170. West D.C., Hampson I.N., Arnold F., Kumar S. Angiogenesis induced by degradation products of hyaluronic acid// Science.- 1985.-V.228.- P.1324-1326.
171. Wulf HC, Sandby-Mоller J, Kobayasi T, Gniadecki R. Skin aging and natural photoprotection. Micron. - 2004. – 35(3):185-91.
172. Zaman A., Cui Z., Foley J. P. et al. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol. - 2005. - 33:447—454.
173. Zouboulis C. C., Adjaye J., Akamatsu H. et al. Human skin stem cells and the aging process . Exp. Geront. - 2008. - Vol. 43.: 986–997.